DNA-dependent protein kinase (DNA-PK) is a nuclear protein Serine (Ser)/Threonine (Thr) kinase that acts as both a molecular sensor and transmitter of DNA damage, and plays important roles in the DNA repair of double stranded breaks (DSBs), mediating immunoglobulin V(D)J gene recombination events, as well as telomere stabilization. [show the full text]
DNA-PK remmers (DNA-PK Inhibitors)
| Cat.nr. | Productnaam | Informatie | Productgebruik Citaten | Productvalidaties |
|---|---|---|---|---|
| S8586 | Nedisertib (M3814) | Nedisertib (M3814, Peposertib, MSC2490484A) is een oraal biologisch beschikbare, zeer krachtige en selectieve remmer van DNA activated protein kinase (DNA-PK) met een IC50 van < 3 nM. |
|
|
| S8843 | AZD7648 | AZD7648 is een krachtige remmer van DNA-PK met een IC50 van 0,6 nM in een biochemische test en meer dan 100-voudig selectief tegen 396 andere kinasen. |
|
|
| S2638 | NU7441 (KU-57788) | NU7441 (KU-57788) is een zeer potente en selectieve DNA-PK-remmer met een IC50 van 14 nM. Het remt ook mTOR en PI3K met een IC50 van respectievelijk 1,7 μM en 5 μM in celvrije assays, en vermindert de frequentie van NHEJ terwijl het de snelheid van HDR verhoogt na Cas9-gemedieerde DNA-splitsing. |
|

|
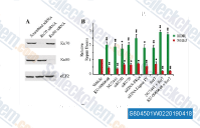
| S8045 | KU-0060648 | KU-0060648 is een dubbele remmer van DNA-PK en PI3Kα, PI3Kβ, PI3Kδ met een IC50 van respectievelijk 8,6 nM en 4 nM, 0,5 nM, 0,1 nM, minder remming van PI3Kγ met een IC50 van 0,59 μM. |
|
|
| S8593 | VX-984 | VX-984 (M9831) is een oraal actieve, potente, selectieve en ATP-competitieve remmer van DNA-PK. Deze verbinding onderdrukt effectief niet-homologe eindverbinding (NHEJ) en verhoogt DNA-dubbelstrengsbreuken (DSB's). Het verbetert de cytotoxische effecten van ioniserende straling (IR) in verschillende kankercellijnen, waaronder niet-kleincellige longkankercellijnen (NSCLC), in vitro. Bovendien vermindert deze remmer de autofosforylering van DNA-PKcs. | ||
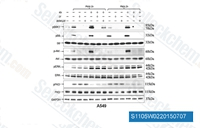
| S1105 | LY294002 | LY294002 (SF 1101, NSC 697286) is de eerste synthetische molecule die PI3Kα/δ/β remt met een IC50 van respectievelijk 0,5 μM/0,57 μM/0,97 μM; stabieler in oplossing dan Wortmannine, en blokkeert ook de vorming van autofagosomen. Het bindt niet alleen aan klasse I PI3K's en andere PI3K-gerelateerde kinasen, maar ook aan nieuwe doelwitten die schijnbaar niet gerelateerd zijn aan de PI3K-familie. Deze verbinding remt ook CK2 met een IC50 van 98 nM. Het is een niet-specifieke DNA-PKcs-remmer en activeert autophagy en apoptosis. |
|
|
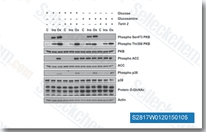
| S2817 | Torin 2 | Torin 2 is een potente en selectieve mTOR-remmer met een IC50 van 0,25 nM in de p53−/− MEFs-cellijn; 800-voudig grotere selectiviteit voor mTOR dan PI3K en verbeterde farmacokinetische eigenschappen. Deze verbinding remt ATM/ATR/DNA-PK met een EC50 van respectievelijk 28 nM/35 nM/118 nM, in PC3-cellijnen. Het vermindert de cellevensvatbaarheid en induceert autophagy en apoptosis. |
|
|
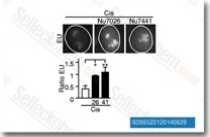
| S2893 | NU7026 | NU7026 (LY293646) is een krachtige DNA-PK-remmer met een IC50 van 0,23 μM in celvrije testen, 60 keer selectiever voor DNA-PK dan PI3K en inactief tegen zowel ATM als ATR. Deze verbinding verhoogt G2/M-celarrest en apoptose. |
|
|
| S7891 | CC-115 | CC-115 is een dubbele remmer van DNA-dependent protein kinase (DNA-PK) en mammalian target of rapamycin (mTOR) met IC50-waarden van respectievelijk 0,013 μM en 0,021 μM. Het heeft een potentiële antineoplastische activiteit. |
|
|
| S8379 | YU238259 | YU238259 is een nieuwe remmer van homologie-afhankelijke DNA-reparatie (HDR), maar remt geen niet-homologe eindverbinding (NHEJ), in celgebaseerde GFP-reporterassays. |
|
|
DNA-dependent protein kinase (DNA-PK) is composed of three key components including two DNA-binding subunits Ku70 and Ku80 (Ku86), as well as one DNA-dependent protein kinase catalytic subunit (DNA-PKcs). Based on protein sequence similarity, DNA-PK belongs to the phosphatidylinositol-3-kinase (PI3K) family, whereas, DNA-PK is not known to phosphorylate lipids and is therefore called PI3K-like kinase (PI3KK). The carboxyl-terminal region of Ku70 contains a SAP domain that is believed to be involved in chromosomal organization. The carboxyl-terminal region of Ku80 is required for the Ku70 and Ku80 heterodimer interaction with DNA-PKcs. The Ku heterodimer can bind to a variety of double-stranded end structures, including blunt ends, overhangs are the 3' or 5' end, and covalently closed hairpin ends. Like ATM and ATR, DNA-PKcs is structurally similar as it contains carboxyl-terminal domains, a large amino-terminal domain in addition to FAT and FATC domains flanking the kinase domain. The DNA-PKcs structure contains a channel large enough to accommodate double-stranded DNA, while the structure of Ku heterodimer is an asymmetric open ring, allowing the DNA to pass through the center. DNA-PKcs is one of the largest kinases identified to date, and it is the only kinase that is absolutely dependent on DNA binding for activity. DNA-PK has a strong preference for phosphorylating Serine (Ser) and Threonine (Thr) residues that are followed by glutamine or, less commonly, a hydrophobic residue. [1][2]
DNA-PK is involved in the ligation step of the non-homologous end joining (NHEJ) pathway required for DNA double-stranded break (DSB) repair, V(D)J recombination and telomere stabilization. A heterodimer of Ku70 and Ku80 initially binds to the double-stranded DNA broken ends and translocates inwards in an ATP-independent manner and recruits DNA-PKcs. This results in the stabilization of the protein/DNA binding and enabling NHEJ to proceed. Moreover, DNA-PKcs acts as a scaffold protein by joining two broken DNA ends together in a complex containing two DNA-PKcs molecules that contributes to the synapsis of the broken DNA ends and the localization of DNA repair proteins such as DNA ligase IV/XRCC4 complex to the site of damage. DNA-PK is activated in cis by the DNA to which it is bound, and stimulated by Ku heterodimer as well as the interaction of two molecules of DNA-PKcs, while end-bridging through synapsis is required for full kinase activation. DNA-PKcs autophosphorylation at multiple sites, including Thr2609 and Ser2056, results in an inactivation of DNA-PK kinase activity and NHEJ ability. To ensure NHEJ can proceed efficiently, DNA-PK phosphorylates and activates the Werner syndrome protein (WRN) to remove 3' phosphate or 3' phosphoglycolate groups generated following IR, and the nuclease Artemis to remove 5' overhangs and shorten 3' overhangs. In addition, DNA-PK promotes processing of hairpin DNA structures in V(D)J recombination by activation of Artemis. Cells that lack DNA-PKcs are acutely radiosensitive and have defective DSB repair, while mice lacking DNA-PKcs remain viable but are immunodeficient (due to the absence of immune development) as a result of accumulated processed DNA intermediates. Additionally, DNA-PK has been strongly implicated in telomere maintenance. DNA-PKcs-/- mice display significant telomeric fusion events consistent with the role of DNA-PKcs in telomere maintenance. Furthermore, DNA-PK is involved in the modulation of transcription by phosphorylation of RNA polymerases including pol I and pol II through its kinase activity, thereby regulating the function of these enzymes. By direct p53 phosphorylation, the modification of Ku70 releasing Bax, or suppressing the expression of p21, DNA-PK plays a significant role in mediating a p53-dependent apoptotic response under a range of cellular conditions including exposure to ionizing radiation (IR), environmental carcinogens and chemotherapeutic agents or in cells that have critically shortened telomeres. [1][2]
Specific inhibitors of DNA-PK used to selectively reduce NHEJ activity have been shown to be effective as single-agent therapies in homologous recombination (HR) -defective tumors. Treatment with a flavone-based DNA-PK inhibitor IC87361 leads to tumor regression. The inhibitors of DNA-PK such as NU7441 enhance the cytotoxicity of physical and chemical agents, leading to reduced clonogenic survival and cellular proliferation, as well as increased apoptosis, regardless of p53 status. Moreover, DNA-PK inhibitors combined with other DNA-damage response (DDR) inhibitors enhance the therapeutic potential of anticancer agents. [3][4]
