연구용
CaMKII-α Antibody [P6K24]
카탈로그 번호: F4909
적용:
반응성:
실험 필수품
WB
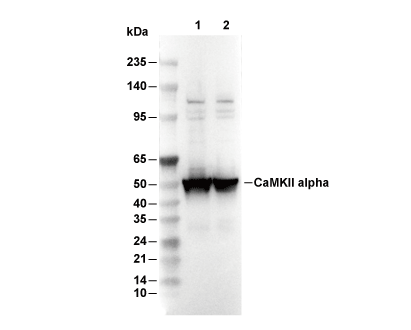
Recommended WB dilution ratio: 1:200
Recommended WB dilution ratio: 1:200
사용 정보
| 희석 |
|---|
|
| 응용 |
|---|
| WB, IHC |
| 반응성 |
|---|
| Mouse, Rat, Human |
| 출처 |
|---|
| Mouse Monoclonal Antibody |
| 보관 완충액 |
|---|
| PBS, pH 7.2+50% Glycerol+0.05% BSA+0.01% NaN3 |
| 보관 (수령일로부터) |
|---|
| -20°C (avoid freeze-thaw cycles), 2 years |
| 예측 분자량 관찰 분자량 |
|---|
| 54 kDa 100 kDa, 50-70 kDa |
| *예측 분자량과 실제 분자량이 다른 이유는 무엇입니까? 다음과 같은 이유로 예측 분자량과 실제 단백질 분자량 간에 차이가 있을 수 있습니다. |
| 양성 대조군 | Human brain tissue; Rat brain tissue; Mouse brain tissue; Rat normal brain (Purkinje cells, cerebellum) |
|---|---|
| 음성 대조군 |
실험 방법
| WB |
|---|
Experimental Protocol:
Sample preparation
1. Tissue: Lyse the tissue sample by adding an appropriate volume of ice-cold RIPA/NP-40 Lysis Buffer (containing Protease Inhibitor Cocktail),and homogenize the tissue at a low temperature. 2. Adherent cell: Aspirate the culture medium and wash the cells with ice-cold PBS twice. Lyse the cells by adding an appropriate volume of RIPA/NP-40 Lysis Buffer (containing Protease Inhibitor Cocktail) and put the sample on ice for 5 min. 3. Suspension cell: Transfer the culture medium to a pre-cooled centrifuge tube. Centrifuge and aspirate the supernatant. Wash the cells with ice-cold PBS twice. Lyse the cells by adding an appropriate volume of RIPA/NP-40 Lysis Buffer (containing Protease Inhibitor Cocktail) and put the sample on ice for 5 min. 4. Place the lysate into a pre-cooled microcentrifuge tube. Centrifuge at 4°C for 15 min. Collect the supernatant;
5. Remove a small volume of lysate to determine the protein concentration;
6. Combine the lysate with protein loading buffer. Boil 20 µL sample under 95-100°C for 5 min. Centrifuge for 5 min after cool down on ice.
Electrophoretic separation
1. According to the concentration of extracted protein, load appropriate amount of protein sample and marker onto SDS-PAGE gels for electrophoresis. Recommended separating gel (lower gel) concentration: 10%. Reference Table for Selecting SDS-PAGE Separation Gel Concentrations 2. Power up 80V for 30 minutes. Then the power supply is adjusted (110 V~150 V), the Marker is observed, and the electrophoresis can be stopped when the indicator band of the predyed protein Marker where the protein is located is properly separated. (Note that the current should not be too large when electrophoresis, too large current (more than 150 mA) will cause the temperature to rise, affecting the result of running glue. If high currents cannot be avoided, an ice bath can be used to cool the bath.)
Transfer membrane
1. Take out the converter, soak the clip and consumables in the pre-cooled converter;
2. Activate PVDF membrane with methanol for 1 min and rinse with transfer buffer;
3. Install it in the order of "black edge of clip - sponge - filter paper - filter paper - glue -PVDF membrane - filter paper - filter paper - sponge - white edge of clip"; 4. The protein was electrotransferred to PVDF membrane. ( 0.45 µm PVDF membrane is recommended ) Reference Table for Selecting PVDF Membrane Pore Size Specifications Recommended conditions for wet transfer: 200 mA, 120 min. ( Note that the transfer conditions can be adjusted according to the protein size. For high-molecular-weight proteins, a higher current and longer transfer time are recommended. However, ensure that the transfer tank remains at a low temperature to prevent gel melting.)
Block
1. After electrotransfer, wash the film with TBST at room temperature for 5 minutes;
2. Incubate the film in the blocking solution for 1 hour at room temperature;
3. Wash the film with TBST for 3 times, 5 minutes each time.
Antibody incubation
1. Use 5% skim milk powder to prepare the primary antibody working liquid (recommended dilution ratio for primary antibody 1:200), gently shake and incubate with the film at 4°C overnight; 2. Wash the film with TBST 3 times, 5 minutes each time;
3. Add the secondary antibody to the blocking solution and incubate with the film gently at room temperature for 1 hour;
4. After incubation, wash the film with TBST 3 times for 5 minutes each time.
Antibody staining
1. Add the prepared ECL luminescent substrate (or select other color developing substrate according to the second antibody) and mix evenly;
2. Incubate with the film for 1 minute, remove excess substrate (keep the film moist), wrap with plastic film, and expose in the imaging system. |
| IHC |
|---|
Experimental Protocol:
Deparaffinization/Rehydration
1. Deparaffinize/hydrate sections:
2. Incubate sections in three washes of xylene for 5 min each.
3. Incubate sections in two washes of 100% ethanol for 10 min each.
4. Incubate sections in two washes of 95% ethanol for 10 min each.
5. Wash sections two times in dH2O for 5 min each.
6.Antigen retrieval: For Citrate: Heat slides in a microwave submersed in 1X citrate unmasking solution until boiling is initiated; continue with 10 min at a sub-boiling temperature (95°-98°C). Cool slides on bench top for 30 min.
Staining
1. Wash sections in dH2O three times for 5 min each.
2. Incubate sections in 3% hydrogen peroxide for 10 min.
3. Wash sections in dH2O two times for 5 min each.
4. Wash sections in wash buffer for 5 min.
5. Block each section with 100–400 µl of blocking solution for 1 hr at room temperature.
6. Remove blocking solution and add 100–400 µl primary antibody diluent in to each section. Incubate overnight at 4°C.
7. Remove antibody solution and wash sections with wash buffer three times for 5 min each.
8. Cover section with 1–3 drops HRPas needed. Incubate in a humidified chamber for 30 min at room temperature.
9. Wash sections three times with wash buffer for 5 min each.
10. Add DAB Chromogen Concentrate to DAB Diluent and mix well before use.
11. Apply 100–400 µl DAB to each section and monitor closely. 1–10 min generally provides an acceptable staining intensity.
12. Immerse slides in dH2O.
13. If desired, counterstain sections with hematoxylin.
14. Wash sections in dH2O two times for 5 min each.
15. Dehydrate sections: Incubate sections in 95% ethanol two times for 10 sec each; Repeat in 100% ethanol, incubating sections two times for 10 sec each; Repeat in xylene, incubating sections two times for 10 sec each.
16. Mount sections with coverslips and mounting medium.
|
생물학적 설명
| 특이성 |
|---|
| CaMKII-α Antibody [P6K24] detects endogenous levels of total CaMKII-α protein. |
| 세포 내 위치 |
|---|
| Cell projection, Synapse |
| Uniprot ID |
|---|
| Q9UQM7 |
| 클론 |
|---|
| P6K24 |
| 동의어 |
|---|
| CAMKA; KIAA0968; CAMK2A; Calcium/calmodulin-dependent protein kinase type II subunit alpha; CaM kinase II subunit alpha; CaMK-II subunit alpha |
| 배경 |
|---|
| CaMKII-α (calcium/calmodulin-dependent protein kinase II alpha) is the principal neuronal isoform of the CaMKII serine/threonine kinase family and assembles into dodecameric or tetradecameric holoenzymes that are pivotal for synaptic plasticity and memory formation. CaMKII-α contains an N-terminal bilobal kinase domain (residues 1-270) where conserved Asp156 and Lys42 coordinate ATP in the catalytic cleft, an autoinhibitory regulatory domain (271-315) with a pseudosubstrate sequence in which Arg-to-Thr286 mimics substrate occlusion and blocks access until displaced by Ca²⁺/calmodulin (CaM), a variable linker region that allows for flexible kinase positioning, and a C-terminal hub/association domain (316-478) that forms a rigid toroidal β-sheet scaffold via four-helix bundles per subunit, supporting intersubunit allosteric interactions. Upon Ca²⁺/CaM binding, the regulatory domain is displaced, relieving autoinhibition and exposing Thr286 for intersubunit trans-autophosphorylation, which prevents CaM dissociation (the "CaM trap") and confers Ca²⁺-independent activity that can persist for over an hour. This mechanism enables CaMKII-α to decode Ca²⁺ oscillation frequency by sequential, wave-like phosphorylation that propagates bidirectionally around the holoenzyme ring, amplifying signaling events such as GluA1 Ser831 phosphorylation to drive AMPAR trafficking and long-term potentiation (LTP). Autophosphorylation at Thr305/306 or oxidation (Met281/282 sulfenic acid formation) further modulates autoinhibition recapture, while phosphatases PP1 and PP2A reverse Thr286 phosphorylation, a process that can be inhibited by PKA crosstalk. Pathologically, hyperactivity of α-CaMKII due to T286I mutation or SCN1A-linked disinhibition leads to epilepsy through hyperexcitable neuronal networks and dendritic defects; heterozygous knockout impairs hippocampal LTP and spatial memory, but can confer resilience to neurodegeneration. Additionally, isoform imbalance disrupts CaV1.2 channel coupling, contributing to cardiac arrhythmias. |
| 참고문헌 |
|---|
|