연구용
CXCL12/SDF-1 Antibody [N2D21]
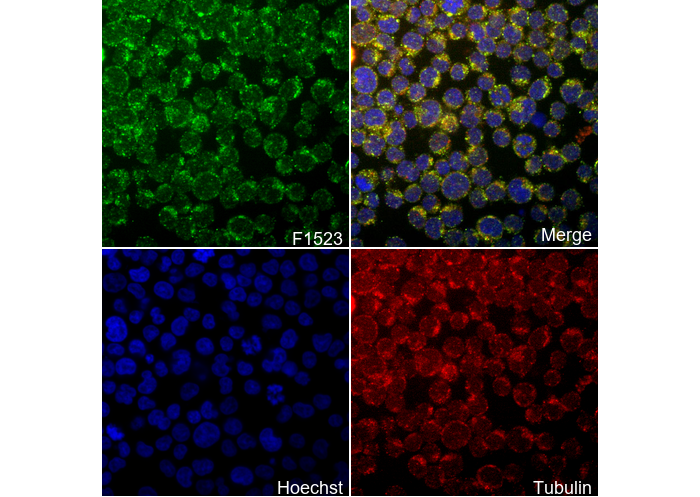
카탈로그 번호: F1523
사용 정보
| 희석 |
|---|
|
| 응용 |
|---|
| IHC, IF, FCM, CyTOF-ready |
| 반응성 |
|---|
| Human, Mouse |
| 출처 |
|---|
| Mouse Monoclonal Antibody |
| 보관 완충액 |
|---|
| PBS, pH 7.2+50% Glycerol+0.05% BSA+0.01% NaN₃ |
| 보관 (수령일로부터) |
|---|
| –20°C (avoid freeze-thaw cycles), 2 years |
| 예측 분자량 |
|---|
| 10 kDa |
| 양성 대조군 | Human PBMCs; Humn tonsil; HT1080 |
|---|---|
| 음성 대조군 |
실험 방법
| IF |
|---|
Experimental Protocol:
Sample Preparation
1. Adherent Cells: Place a clean, sterile coverslip in a culture dish. Once the cells grow to near confluence as a monolayer, remove the coverslip for further use.
2. Suspension Cells: Seed the cells onto a clean, sterile slide coated with poly-L-lysine.
3. Frozen Sections: Allow the slide to thaw at room temperature. Wash it with pure water or PBS for 2 times, 3 minutes each time.
4. Paraffin Sections: Deparaffinization and rehydration. Wash the slide with pure water or PBS for 3 times, 3 minutes each time. Then perform antigen retrieval.
Fixation
1. Fix the cell coverslips/spots or tissue sections at room temperature using a fixative such as 4% paraformaldehyde (4% PFA) for 10-15 minutes.
2. Wash the sample with PBS for 3 times, 3 minutes each time.
Permeabilization
1.Add a detergent such as 0.1–0.3% Triton X-100 to the sample and incubate at room temperature for 10–20 minutes.
(Note: This step is only required for intracellular antigens. For antigens expressed on the cell membrane, this step is unnecessary.)
Wash the sample with PBS for 3 times, 3 minutes each time.
Blocking
Add blocking solution and incubate at room temperature for at least 1 hour. (Common blocking solutions include: serum from the same source as the secondary antibody, BSA, or goat serum.)
Note: Ensure the sample remains moist during and after the blocking step to prevent drying, which can lead to high background.
Immunofluorescence Staining (Day 1)
1. Remove the blocking solution and add the diluted primary antibody.
2. Incubate the sample in a humidified chamber at 4°C overnight.
Immunofluorescence Staining (Day 2)
1. Remove the primary antibody and wash with PBST for 3 times, 5 minutes each time.
2. Add the diluted fluorescent secondary antibody and incubate in the dark at 4°C for 1–2 hours.
3. Remove the secondary antibody and wash with PBST for 3 times, 5 minutes each time.
4. Add diluted DAPI and incubate at room temperature in the dark for 5–10 minutes.
5. Wash with PBST for 3 times, 5 minutes each time.
Mounting
1. Mount the sample with an anti-fade mounting medium.
2. Allow the slide to dry at room temperature overnight in the dark.
3. Store the slide in a slide storage box at 4°C, protected from light.
|
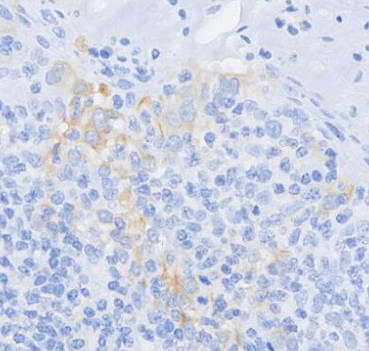
| IHC |
|---|
Experimental Protocol: Deparaffinization/Rehydration
1. Deparaffinize/hydrate sections:
2. Incubate sections in three washes of xylene for 5 min each.
3. Incubate sections in two washes of 100% ethanol for 10 min each.
4. Incubate sections in two washes of 95% ethanol for 10 min each.
5. Wash sections two times in dH2O for 5 min each.
6.Antigen retrieval: For Citrate: Heat slides in a microwave submersed in 1X citrate unmasking solution until boiling is initiated; continue with 10 min at a sub-boiling temperature (95°-98°C). Cool slides on bench top for 30 min.
Staining
1. Wash sections in dH2O three times for 5 min each.
2. Incubate sections in 3% hydrogen peroxide for 10 min.
3. Wash sections in dH2O two times for 5 min each.
4. Wash sections in wash buffer for 5 min.
5. Block each section with 100–400 µl of blocking solution for 1 hr at room temperature.
6. Remove blocking solution and add 100–400 µl primary antibody diluent in to each section. Incubate overnight at 4°C.
7. Remove antibody solution and wash sections with wash buffer three times for 5 min each.
8. Cover section with 1–3 drops HRPas needed. Incubate in a humidified chamber for 30 min at room temperature.
9. Wash sections three times with wash buffer for 5 min each.
10. Add DAB Chromogen Concentrate to DAB Diluent and mix well before use.
11. Apply 100–400 µl DAB to each section and monitor closely. 1–10 min generally provides an acceptable staining intensity.
12. Immerse slides in dH2O.
13. If desired, counterstain sections with hematoxylin.
14. Wash sections in dH2O two times for 5 min each.
15. Dehydrate sections: Incubate sections in 95% ethanol two times for 10 sec each; Repeat in 100% ethanol, incubating sections two times for 10 sec each; Repeat in xylene, incubating sections two times for 10 sec each.
16. Mount sections with coverslips and mounting medium.
|
생물학적 설명
| 특이성 |
|---|
CXCL12/SDF-1 Antibody [N2D21] detects endogenous levels of total CXCL12/SDF-1 protein. |
| 세포 내 위치 |
|---|
| Secreted |
| Uniprot ID |
|---|
| Q6ICW0 |
| 클론 |
|---|
| N2D21 |
| 동의어 |
|---|
| SDF-1,CXCL12 |
| 배경 |
|---|
CXCL12, also known as Stromal-cell-derived factor-1 (SDF-1), is a chemokine involved in numerous physiological processes, including embryonic development, organ homeostasis, leukocyte migration, stem cell homing, angiogenic activity and cancer metastasis. It exists in multiple splice variants, primarily SDF-1α and SDF-1β, SDF-1γ, expressed in the nervous system. CXCL12 forms a monomer–dimer equilibrium that shifts toward a dimer upon crystallization or binding to its receptor, CXCR4. CXCR4, the primary receptor for CXCL12, is crucial for fetal development and acts as a co-receptor for T-tropic HIV-1, where CXCL12 inhibits viral entry. CXCL12 expression is upregulated in ischemic conditions (e.g., in the heart and skeletal muscle) and has a protective role after myocardial infarction, making it a potential target for anti-ischemic therapies. In cancer, CXCL12/CXCR4 signaling promotes cancer cell migration and metastasis, with CXCR4 inhibition shown to reduce tumor metastasis in experimental models. |
| 참고문헌 |
|---|
|