연구용
Peroxiredoxin 6 Antibody [G12J13]
카탈로그 번호: F2838
적용:
반응성:
실험 필수품
WB
Recommended wet transfer conditions: 200 mA, 60 min.
Recommended wet transfer conditions: 200 mA, 60 min.
사용 정보
| 희석 |
|---|
|
| 응용 |
|---|
| WB, IHC |
| 반응성 |
|---|
| Mouse, Rat, Human |
| 출처 |
|---|
| Rabbit Monoclonal Antibody |
| 보관 완충액 |
|---|
| PBS, pH 7.2+50% Glycerol+0.05% BSA+0.01% NaN3 |
| 보관 (수령일로부터) |
|---|
| -20°C (avoid freeze-thaw cycles), 2 years |
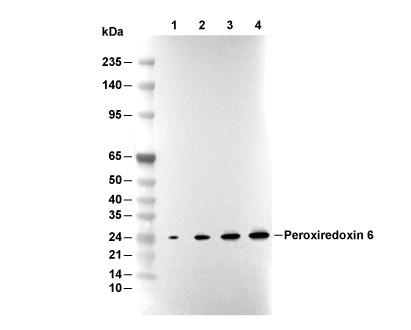
| 예측 분자량 관찰 분자량 |
|---|
| 25 kDa 25 kDa |
| *예측 분자량과 실제 분자량이 다른 이유는 무엇입니까? 다음과 같은 이유로 예측 분자량과 실제 단백질 분자량 간에 차이가 있을 수 있습니다. |
| 양성 대조군 | Human stomach tissue; Human placenta tissue; Human testis tissue; Human brain tissue; Human liver tissue; Fetal liver tissue; Mouse liver tissue; Mouse testis tissue; Mouse spleen tissue; Rat liver tissue; Rat testis tissue; Rat spleen tissue; HAP1 cells; A431 cells; PC3 cells; HeLa cells; 293 cells |
|---|---|
| 음성 대조군 |
실험 방법
| WB |
|---|
Experimental Protocol:
Sample preparation
1. Tissue: Lyse the tissue sample by adding an appropriate volume of ice-cold RIPA/NP-40 Lysis Buffer (containing Protease Inhibitor Cocktail),and homogenize the tissue at a low temperature. 2. Adherent cell: Aspirate the culture medium and wash the cells with ice-cold PBS twice. Lyse the cells by adding an appropriate volume of RIPA/NP-40 Lysis Buffer (containing Protease Inhibitor Cocktail) and put the sample on ice for 5 min. 3. Suspension cell: Transfer the culture medium to a pre-cooled centrifuge tube. Centrifuge and aspirate the supernatant. Wash the cells with ice-cold PBS twice. Lyse the cells by adding an appropriate volume of RIPA/NP-40 Lysis Buffer (containing Protease Inhibitor Cocktail) and put the sample on ice for 5 min. 4. Place the lysate into a pre-cooled microcentrifuge tube. Centrifuge at 4°C for 15 min. Collect the supernatant;
5. Remove a small volume of lysate to determine the protein concentration;
6. Combine the lysate with protein loading buffer. Boil 20 µL sample under 95-100°C for 5 min. Centrifuge for 5 min after cool down on ice.
Electrophoretic separation
1. According to the concentration of extracted protein, load appropriate amount of protein sample and marker onto SDS-PAGE gels for electrophoresis. Recommended separating gel (lower gel) concentration: 10%. Reference Table for Selecting SDS-PAGE Separation Gel Concentrations 2. Power up 80V for 30 minutes. Then the power supply is adjusted (110 V~150 V), the Marker is observed, and the electrophoresis can be stopped when the indicator band of the predyed protein Marker where the protein is located is properly separated. (Note that the current should not be too large when electrophoresis, too large current (more than 150 mA) will cause the temperature to rise, affecting the result of running glue. If high currents cannot be avoided, an ice bath can be used to cool the bath.)
Transfer membrane
1. Take out the converter, soak the clip and consumables in the pre-cooled converter;
2. Activate PVDF membrane with methanol for 1 min and rinse with transfer buffer;
3. Install it in the order of "black edge of clip - sponge - filter paper - filter paper - glue -PVDF membrane - filter paper - filter paper - sponge - white edge of clip"; 4. The protein was electrotransferred to PVDF membrane. ( 0.45 µm PVDF membrane is recommended ) Reference Table for Selecting PVDF Membrane Pore Size Specifications Recommended conditions for wet transfer: 200 mA, 60 min. ( Note that the transfer conditions can be adjusted according to the protein size. For high-molecular-weight proteins, a higher current and longer transfer time are recommended. However, ensure that the transfer tank remains at a low temperature to prevent gel melting.)
Block
1. After electrotransfer, wash the film with TBST at room temperature for 5 minutes;
2. Incubate the film in the blocking solution for 1 hour at room temperature;
3. Wash the film with TBST for 3 times, 5 minutes each time.
Antibody incubation
1. Use 5% skim milk powder to prepare the primary antibody working liquid (recommended dilution ratio for primary antibody 1:1000), gently shake and incubate with the film at 4°C overnight; 2. Wash the film with TBST 3 times, 5 minutes each time;
3. Add the secondary antibody to the blocking solution and incubate with the film gently at room temperature for 1 hour;
4. After incubation, wash the film with TBST 3 times for 5 minutes each time.
Antibody staining
1. Add the prepared ECL luminescent substrate (or select other color developing substrate according to the second antibody) and mix evenly;
2. Incubate with the film for 1 minute, remove excess substrate (keep the film moist), wrap with plastic film, and expose in the imaging system. |
| IHC |
|---|
Experimental Protocol:
Deparaffinization/Rehydration
1. Deparaffinize/hydrate sections:
2. Incubate sections in three washes of xylene for 5 min each.
3. Incubate sections in two washes of 100% ethanol for 10 min each.
4. Incubate sections in two washes of 95% ethanol for 10 min each.
5. Wash sections two times in dH2O for 5 min each.
6.Antigen retrieval: For Citrate: Heat slides in a microwave submersed in 1X citrate unmasking solution until boiling is initiated; continue with 10 min at a sub-boiling temperature (95°-98°C). Cool slides on bench top for 30 min.
Staining
1. Wash sections in dH2O three times for 5 min each.
2. Incubate sections in 3% hydrogen peroxide for 10 min.
3. Wash sections in dH2O two times for 5 min each.
4. Wash sections in wash buffer for 5 min.
5. Block each section with 100–400 µl of blocking solution for 1 hr at room temperature.
6. Remove blocking solution and add 100–400 µl primary antibody diluent in to each section. Incubate overnight at 4°C.
7. Remove antibody solution and wash sections with wash buffer three times for 5 min each.
8. Cover section with 1–3 drops HRPas needed. Incubate in a humidified chamber for 30 min at room temperature.
9. Wash sections three times with wash buffer for 5 min each.
10. Add DAB Chromogen Concentrate to DAB Diluent and mix well before use.
11. Apply 100–400 µl DAB to each section and monitor closely. 1–10 min generally provides an acceptable staining intensity.
12. Immerse slides in dH2O.
13. If desired, counterstain sections with hematoxylin.
14. Wash sections in dH2O two times for 5 min each.
15. Dehydrate sections: Incubate sections in 95% ethanol two times for 10 sec each; Repeat in 100% ethanol, incubating sections two times for 10 sec each; Repeat in xylene, incubating sections two times for 10 sec each.
16. Mount sections with coverslips and mounting medium.
|
생물학적 설명
| 특이성 |
|---|
| Peroxiredoxin 6 Antibody [G12J13] detects endogenous levels of total Peroxiredoxin 6 protein. |
| 세포 내 위치 |
|---|
| Cytoplasm, Lysosome |
| Uniprot ID |
|---|
| P30041 |
| 클론 |
|---|
| G12J13 |
| 동의어 |
|---|
| AOP2; KIAA0106; PRDX6; Peroxiredoxin-6; 1-Cys peroxiredoxin; 24 kDa protein; Acidic calcium-independent phospholipase A2; Liver 2D page spot 40; 1-Cys PRX; aiPLA2; LPC acyltransferase 5; LPCAT-5; Lyso-PC acyltransferase 5; NSGPx |
| 배경 |
|---|
| Peroxiredoxin 6 (Prdx6) is a distinctive 25 kDa 1-Cys peroxiredoxin expressed in virtually all tissues but highly enriched in the lung, uniquely combining robust peroxidase and phospholipase A2 (PLA2)-like activities within a single 224-amino-acid polypeptide. Its N-terminal thioredoxin fold domain harbors the peroxidatic Cys47 within a hydrophobic pocket, enabling efficient reduction of H₂O₂, short-chain organic, and phospholipid hydroperoxides using glutathione (GSH) as the main reductant, while the distinct surface PLA2 catalytic site (His26-Ser32-Asp140 triad and GDSWG motif) hydrolyzes sn-2 acyl chains from oxidized phospholipids. Prdx6 alternates between reduced monomers (favoring peroxidase activity) and oxidized dimers (enhancing PLA2 function), allowing rapid adaptation to oxidative and lipid stress. Through this dual mechanism, Prdx6 not only safeguards redox balance and membrane integrity, especially in lung surfactant metabolism, but also facilitates full repair by coupling PLA2-mediated phospholipid hydrolysis with lysophosphatidylcholine acyltransferase (LPCAT) reacylation. Prdx6 modulates H₂O₂ signaling and activates NADPH oxidase 2 (Nox2) for inflammatory responses, and cross-talks with thioredoxin networks. Its regulation involves Nrf2-driven transcription, pH-dependent activity switching (neutral for peroxidase, acidic for PLA2), phosphorylation, and glutathionylation, with stress-induced translocation to mitochondria, lysosomes, or plasma membrane. Dysregulation of Prdx6 is linked to multiple pathologies, including cancer (via NF-κB-driven growth, invasion, and therapy resistance), neurodegeneration (influencing both oxidative injury and Tau aggregation), inflammation, osteoporosis, diabetes, cataracts, and infertility. |
| 참고문헌 |
|---|
|