연구용
Phospho-BTK (Tyr223) Antibody [H3N9]
카탈로그 번호: F0977
적용:
반응성:
사용 정보
| 희석 |
|---|
|
| 응용 |
|---|
| WB, IP |
| 반응성 |
|---|
| Human |
| 출처 |
|---|
| Rabbit Monoclonal Antibody |
| 보관 완충액 |
|---|
| PBS, pH 7.2+50% Glycerol+0.05% BSA+0.01% NaN3 |
| 보관 (수령일로부터) |
|---|
| -20°C (avoid freeze-thaw cycles), 2 years |
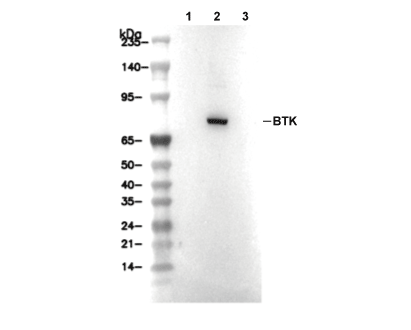
| 예측 분자량 관찰 분자량 |
|---|
| 76 kDa 76 kDa |
| *예측 분자량과 실제 분자량이 다른 이유는 무엇입니까? 다음과 같은 이유로 예측 분자량과 실제 단백질 분자량 간에 차이가 있을 수 있습니다. |
| 양성 대조군 | Ramos cells (Pervanadate, 1mM, 30 min); K562 cells (Pervanadate-treated) |
|---|---|
| 음성 대조군 | Ramos cells; K562 cells |
실험 방법
| WB |
|---|
Experimental Protocol:
Sample preparation
1. Tissue: Lyse the tissue sample by adding an appropriate volume of ice-cold RIPA/NP-40 Lysis Buffer (containing Protease Inhibitor Cocktail, Phosphatase Inhibitor Cocktail),and homogenize the tissue at a low temperature. 2. Adherent cell: Aspirate the culture medium and wash the cells with ice-cold PBS twice. Lyse the cells by adding an appropriate volume of RIPA/NP-40 Lysis Buffer (containing Protease Inhibitor Cocktail, Phosphatase Inhibitor Cocktail) and put the sample on ice for 5 min. 3. Suspension cell: Transfer the culture medium to a pre-cooled centrifuge tube. Centrifuge and aspirate the supernatant. Wash the cells with ice-cold PBS twice. Lyse the cells by adding an appropriate volume of RIPA/NP-40 Lysis Buffer (containing Protease Inhibitor Cocktail, Phosphatase Inhibitor Cocktail) and put the sample on ice for 5 min. 4. Place the lysate into a pre-cooled microcentrifuge tube. Centrifuge at 4°C for 15 min. Collect the supernatant;
5. Remove a small volume of lysate to determine the protein concentration;
6. Combine the lysate with protein loading buffer. Boil 20 µL sample under 95-100°C for 5 min. Centrifuge for 5 min after cool down on ice.
Electrophoretic separation
1. According to the concentration of extracted protein, load appropriate amount of protein sample and marker onto SDS-PAGE gels for electrophoresis. Recommended separating gel (lower gel) concentration: 10%. Reference Table for Selecting SDS-PAGE Separation Gel Concentrations 2. Power up 80V for 30 minutes. Then the power supply is adjusted (110 V~150 V), the Marker is observed, and the electrophoresis can be stopped when the indicator band of the predyed protein Marker where the protein is located is properly separated. (Note that the current should not be too large when electrophoresis, too large current (more than 150 mA) will cause the temperature to rise, affecting the result of running glue. If high currents cannot be avoided, an ice bath can be used to cool the bath.)
Transfer membrane
1. Take out the converter, soak the clip and consumables in the pre-cooled converter;
2. Activate PVDF membrane with methanol for 1 min and rinse with transfer buffer;
3. Install it in the order of "black edge of clip - sponge - filter paper - filter paper - glue -PVDF membrane - filter paper - filter paper - sponge - white edge of clip"; 4. The protein was electrotransferred to PVDF membrane. ( 0.45 µm PVDF membrane is recommended ) Reference Table for Selecting PVDF Membrane Pore Size Specifications Recommended conditions for wet transfer: 200 mA, 120 min. ( Note that the transfer conditions can be adjusted according to the protein size. For high-molecular-weight proteins, a higher current and longer transfer time are recommended. However, ensure that the transfer tank remains at a low temperature to prevent gel melting.)
Block
1. After electrotransfer, wash the film with TBST at room temperature for 5 minutes;
2. Incubate the film in the blocking solution ( recommending 5% BSA solution)
for 1 hour at room temperature;
3. Wash the film with TBST for 3 times, 5 minutes each time.
Antibody incubation
1. Use 5% skim milk powder to prepare the primary antibody working liquid (recommended dilution ratio for primary antibody 1:2000), gently shake and incubate with the film at 4°C overnight; 2. Wash the film with TBST 3 times, 5 minutes each time;
3. Add the secondary antibody to the blocking solution and incubate with the film gently at room temperature for 1 hour;
4. After incubation, wash the film with TBST 3 times for 5 minutes each time.
Antibody staining
1. Add the prepared ECL luminescent substrate (or select other color developing substrate according to the second antibody) and mix evenly;
2. Incubate with the film for 1 minute, remove excess substrate (keep the film moist), wrap with plastic film, and expose in the imaging system. |
생물학적 설명
| 특이성 |
|---|
| Phospho-BTK (Tyr223) Antibody [H3N9] detects endogenous levels of total BTK protein only when it is phosphorylated at Tyr223. |
| 세포 내 위치 |
|---|
| Cell membrane, Cytoplasm, Membrane, Nucleus |
| Uniprot ID |
|---|
| Q06187 |
| 클론 |
|---|
| H3N9 |
| 동의어 |
|---|
| AGMX1; ATK; BPK; BTK; Tyrosine-protein kinase BTK; Agammaglobulinemia tyrosine kinase; B-cell progenitor kinase; Bruton tyrosine kinase |
| 배경 |
|---|
| Phospho-BTK (Tyr223) denotes the activated state of Bruton's tyrosine kinase, a TEC family non-receptor tyrosine kinase essential for B-cell receptor signaling and maturation. This protein possesses a pleckstrin homology (PH) domain for PIP3-driven membrane localization, followed by TEC homology, SH3, SH2, and kinase domains. Activation is initiated by Src family kinases such as Lyn phosphorylating BTK at Tyr551 in the kinase domain, which then enables autophosphorylation at Tyr223 within the SH3 domain. This sequential phosphorylation stabilizes BTK’s open, active conformation, boosting kinase activity toward downstream targets like PLCγ2, which in turn drives IP3 and DAG production, calcium flux, and activation of NF-κB and MAPK cascades. These signals are crucial for B-cell proliferation, differentiation, survival, and effective antigen presentation, while PKCβ-mediated phosphorylation at Ser180 provides negative feedback to set signaling thresholds. Persistent phosphorylation at Tyr223 is implicated in B-cell malignancies such as chronic lymphocytic leukemia and lymphoma, making BTK a major target for therapeutics like ibrutinib. Loss-of-function mutations in BTK, meanwhile, result in X-linked agammaglobulinemia. |
| 참고문헌 |
|---|
|