연구용
Berberine chloride Anti-infection 화학물
제품 번호S2271
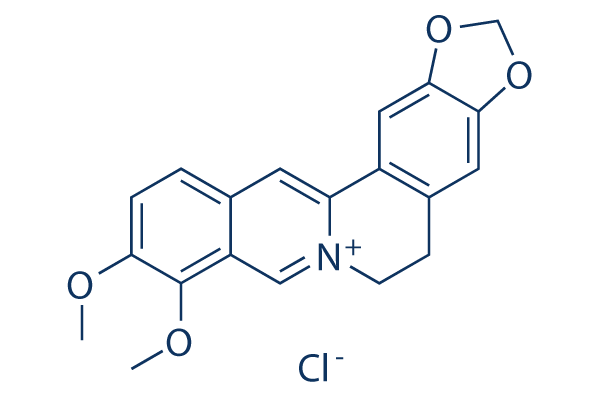
화학 구조
분자량: 371.81
품질 관리
세포 배양, 처리 및 작업 농도
| 세포주 | 분석 유형 | 농도 | 배양 시간 | 제형 | 활성 설명 | PMID |
|---|---|---|---|---|---|---|
| MRC5 cells | Function assay | Antiviral activity against HCMV in MRC5 cells by plaque reduction assay, IC50=0.68 μM | ||||
| MRC5 cells | Proliferation assay | 10 μM | 54 hrs | Inhibition of HCMV proliferation in MRC5 cells after 54 hrs post-infection at 10 uM by plaque assay | ||
| MRC5 cells | Proliferation assay | 10 μM | 24 h | Inhibition of HCMV proliferation in MRC5 cells after 24 hrs post-infection at 10 uM by plaque assay | ||
| Bel7402 cells | Function assay | 12 h | Induction of LDLR protein in human Bel7402 cells after 12 hrs by RT-PCR assay relative to control | |||
| HepG2 cells | Function assay | 10 ug/mL | 12 h | Induction of LDLR protein expression in human HepG2 cells at 10 ug/mL after 12 hrs by flow cytometry | ||
| KB cells | Cytotoxicity assay | 72 h | Cytotoxicity against human KB cells after 72 hrs, IC50=7.32 μM | |||
| HL60 cells | Apoptosis assay | 48 hrs | Induction of apoptosis in human HL60 cells after 48 hrs using annexin V-propidium iodide staining by FACS analysis | |||
| A549 cells | Cytotoxicity assay | Cytotoxicity against human A549 cells by SRB assay, IC50=6.27 μM | ||||
| SKOV3 cells | Cytotoxicity assay | Cytotoxicity against human SKOV3 cells by SRB assay, IC50=16.44 μM | ||||
| SK-MEL-2 cells | Cytotoxicity assay | Cytotoxicity against human SK-MEL-2 cells by SRB assay, IC50=13.76 μM | ||||
| HCT15 cells | Cytotoxicity assay | Cytotoxicity against human HCT15 cells by SRB assay, IC50=16.59 μM | ||||
| CEM cells | Cytotoxicity assay | 48 hrs | Cytotoxicity against human CEM cells expressing green fluorescent protein after 48 hrs by MTT assay, CC50=2.09 μM | |||
| human CEM cells | Function assay | 7 days | Antiviral activity against 0.05 MOI Human immunodeficiency virus 1 NL4.3 infected in human CEM cells expressing green fluorescent protein assessed as p24 antigen production measured 7 days post infection by ELISA, EC50=0.13 μM | |||
| SKN cells | Growth inhibition assay | 72 h | Growth inhibition against human SKN cells after 72 hrs by MTT assay, GI50=15.88 μM | |||
| RKN cells | Growth inhibition assay | 48 hrs | Growth inhibition against human RKN cells after 48 hrs by MTT assay, GI50=49.6 μM | |||
| G402 cells | Growth inhibition assay | 48 hrs | Growth inhibition against human G402 cells after 48 hrs by MTT assay, GI50=11.87 μM | |||
| A10 cells | Function assay | 30 μM | 24 hrs | Downregulation of Scd2 mRNA expression in rat A10 cells at 30 uM after 24 hrs by quantitative RT-PCR analysis | ||
| A10 cells | Function assay | 30 μM | 24 hrs | Down regulation of Prim2 mRNA expression in rat A10 cells at 30 uM after 24 hrs by quantitative RT-PCR analysis | ||
| A10 cells | Function assay | 30 μM | 24 hrs | Downregulation of Impk mRNA expression in rat A10 cells at 30 uM after 24 hrs by quantitative RT-PCR analysis | ||
| HepG2-A16-CD81 cells | Function assay | 10 μM | NOVARTIS: Antimalarial liver stage activity measured as a greater than 50% reduction in Plasmodium yoelii schizont area in HepG2-A16-CD81 cells at 10uM compound concentration, determined by immuno-fluorescence. | |||
| HepG2-A16-CD81 cells | Function assay | 10 μM | NOVARTIS: Antimalarial liver stage activity measured as reduction in Plasmodium yoelii schizont area in HepG2-A16-CD81 cells by immuno-fluorescence, and median schizont size at 10uM compound concentration, IC50=0.548 μM | |||
| HepG2 cells | Function assay | 10 μM | 4 h | Increase in AMPKalpha phosphorylation in human HepG2 cells at 10 uM after 4 hrs by Western blot analysis relative to untreated control | ||
| HepG2 cells | Function assay | 10 μM | 4 h | Increase in total AMPKalpha level in human HepG2 cells at 10 uM after 4 hrs by Western blot analysis relative to untreated control | ||
| HepG2 cells | Function assay | 20 μM | 24 hrs | Induction of apoptosis in human HepG2 cells assessed as morphological changes at 20 uM after 24 hrs using Hoechst 33258 staining by fluorescence microscopic analysis | ||
| HT-29 cells | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HT-29 cells after 48 hrs by MTT assay, IC50=8.45 μM | |||
| HepG2 cells | Cytotoxicity assay | 24 hrs | Cytotoxicity against human HepG2 cells after 24 hrs by MTT assay, IC50=11.22 μM | |||
| HepG2 cells | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells after 48 hrs by MTT assay, IC50=8.32 μM | |||
| 클릭하여 더 많은 세포주 실험 데이터 보기 | ||||||
화학 정보, 보관 및 안정성
| 분자량 | 371.81 | 화학식 | C20H18NO4.Cl |
보관 (수령일로부터) | |
|---|---|---|---|---|---|
| CAS 번호 | 633-65-8 | SDF 다운로드 | 원액 보관 |
|
|
용해도
|
In vitro |
DMSO
: 25 mg/mL
(67.23 mM)
Water : Insoluble Ethanol : Insoluble |
몰농도 계산기
|
In vivo |
|||||
생체 내 제형 계산기 (투명한 용액)
1단계: 아래 정보 입력 (권장: 실험 중 손실을 고려하여 추가 동물 포함)
2단계: 생체 내 제형 입력 (이것은 계산기일 뿐 제형이 아닙니다. 용해도 섹션에 생체 내 제형이 없는 경우 먼저 당사에 문의하십시오.)
계산 결과:
작업 농도: mg/ml;
DMSO 원액 준비 방법: mg 약물 사전 용해 μL DMSO ( 원액 농도 mg/mL, 농도가 해당 약물 배치의 DMSO 용해도를 초과하는 경우 먼저 당사에 문의하십시오. )
생체 내 제형 준비 방법: 취하다 μL DMSO 원액, 다음 추가μL PEG300, 혼합하고 투명하게 한 다음 추가μL Tween 80, 혼합하고 투명하게 한 다음 추가 μL ddH2O, 혼합하고 투명하게 합니다.
생체 내 제형 준비 방법: 취하다 μL DMSO 원액, 다음 추가 μL 옥수수 기름, 혼합하고 투명하게 합니다.
참고: 1. 다음 용매를 추가하기 전에 액체가 투명한지 확인하십시오.
2. 용매를 순서대로 추가해야 합니다. 다음 용매를 추가하기 전에 이전 추가에서 얻은 용액이 투명한 용액인지 확인해야 합니다. 와동, 초음파 또는 뜨거운 물 중탕과 같은 물리적 방법을 사용하여 용해를 도울 수 있습니다.
작용 메커니즘
| Targets/IC50/Ki |
Caspase-3
Caspase-8
PARP
cytochrome c
cIAP1
Bcl-2
Bcl-xL
JNK
p38 MAPK
ROS
Topo I
Topo II
|
|---|---|
| 시험관 내(In vitro) |
레고라페닙 단독 투여와 비교하여, Berberine (BBR)과 레고라페닙의 병용 치료는 간세포암종(HCC) 세포의 증식을 유의하게 억제하고 세포 apoptosis를 유도합니다. |
| 생체 내(In vivo) |
Berberine (BBR)과 레고라페닙의 병용 치료군은 누드 마우스에서 간세포암종(HCC) 이종이식 종양의 성장에 극적인 억제 효과를 나타냅니다. 병용 치료군에서 이종이식 종양의 apoptosis 증가가 관찰됩니다. |
참조 |
|
임상시험 정보
(데이터 출처 https://clinicaltrials.gov, 업데이트 날짜 2024-05-22)
| NCT 번호 | 모집 | 조건 | 스폰서/협력자 | 시작일 | 단계 |
|---|---|---|---|---|---|
| NCT06273241 | Not yet recruiting | Pharmacokinetic Study in Healthy Volunteers |
University Medicine Greifswald |
March 4 2024 | Not Applicable |
| NCT05845931 | Recruiting | Pharmacokinetic Study in Healthy Volunteers |
University Medicine Greifswald |
May 5 2023 | Not Applicable |
| NCT05480670 | Completed | Polycystic Ovary Syndrome |
Ayub Teaching Hospital |
November 1 2022 | Not Applicable |
| NCT05463003 | Completed | Pharmacokinetic Study in Healthy Volunteers |
University Medicine Greifswald |
July 19 2022 | Not Applicable |
