연구용
GS-441524 Antiviral 억제제
제품 번호S6814
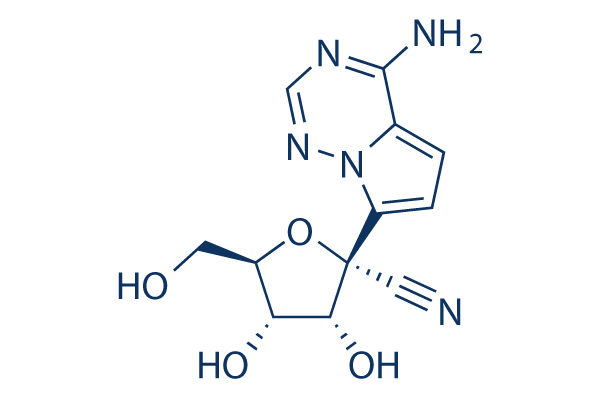
화학 구조
분자량: 291.26
품질 관리
세포 배양, 처리 및 작업 농도
| 세포주 | 분석 유형 | 농도 | 배양 시간 | 제형 | 활성 설명 | PMID |
|---|---|---|---|---|---|---|
| HuH7 | Function assay | 10 uM | 24 hrs | Cmax in human HuH7 cells assessed as triphosphate concentration per million cells at 10 uM incubated for 24 hrs measured after washout by LC-MS analysis, Cmax=0.0000176μM | 22446091 | |
| Vero | Antiviral assay | 24 hrs | Antiviral activity against Parainfluenza 3 C 243 infected in african green monkey Vero cells assessed as inhibition of virus induced cytopathic effect after 24 hrs by MTS assay, EC50=1.71μM | 22446091 | ||
| Vero | Antiviral assay | 24 hrs | Antiviral activity against SARS coronavirus Toronto-2 infected in african green monkey Vero cells assessed as inhibition of virus induced cytopathic effect after 24 hrs by MTS assay, EC50=2.24μM | 22446091 | ||
| HuH7 | Antiviral assay | 24 hrs | Antiviral activity against Hepatitis C virus genotype 1b infected in human HuH7 cells assessed as inhibition of virus induced cytopathic effect after 24 hrs by MTS assay, EC50=4.1μM | 22446091 | ||
| Vero E6 | Antiviral assay | 24 hrs | Antiviral activity against Dengue virus type 2 New Guinea C infected in african green monkey Vero E6 cells assessed as inhibition of virus induced cytopathic effect after 24 hrs by MTS assay, EC50=9.46μM | 22446091 | ||
| HeLa | Antiviral assay | 24 hrs | Antiviral activity against Yellow fever virus 17D infected in human HeLa cells assessed as inhibition of virus induced cytopathic effect after 24 hrs by MTS assay, EC50=11μM | 22446091 | ||
| MDCK | Antiviral assay | 24 hrs | Antiviral activity against Influenza A virus H3N2 infected in MDCK cells assessed as inhibition of virus induced cytopathic effect after 24 hrs by MTS assay, EC50=27.9μM | 22446091 | ||
| Hep2 | Antiviral assay | 4 days | Antiviral activity against Respiratory syncytial virus A2 infected in human Hep2 cells assessed as reduction of virus-induced cytopathic effect after 4 days by Cell-Titer Glo assay, EC50=0.53μM | 28124907 | ||
| HMVEC | Antiviral assay | 3 to 4 days | Antiviral activity against GFP-fused Ebolavirus infected in TERT-immortalized HMVEC assessed as reduction in viral replication preincubated with cells followed by viral infection measured after 3 to 4 days by fluorescence assay, EC50=0.78μM | 28124907 | ||
| HuH7 | Antiviral assay | 3 days | Antiviral activity against Hepatitis C virus genotype 1b infected in human HuH7 cells preincubated with cells for 3 days followed by viral infection by luciferase assay, EC50=4.1μM | 28124907 | ||
| HuH7 | Antiviral assay | 3 days | Antiviral activity against Ebolavirus infected in human HuH7 cells after 3 days by end point dilution assay, EC50=1.5μM | 28792763 | ||
| 클릭하여 더 많은 세포주 실험 데이터 보기 | ||||||
화학 정보, 보관 및 안정성
| 분자량 | 291.26 | 화학식 | C12H13N5O4 |
보관 (수령일로부터) | 3 years -20°C powder |
|---|---|---|---|---|---|
| CAS 번호 | 1191237-69-0 | -- | 원액 보관 |
|
|
| 동의어 | N/A | Smiles | C1=C2C(=NC=NN2C(=C1)C3(C(C(C(O3)CO)O)O)C#N)N | ||
용해도
|
In vitro |
DMSO
: 58 mg/mL
(199.13 mM)
Water : Insoluble Ethanol : Insoluble |
몰농도 계산기
|
In vivo |
|||||
생체 내 제형 계산기 (투명한 용액)
1단계: 아래 정보 입력 (권장: 실험 중 손실을 고려하여 추가 동물 포함)
2단계: 생체 내 제형 입력 (이것은 계산기일 뿐 제형이 아닙니다. 용해도 섹션에 생체 내 제형이 없는 경우 먼저 당사에 문의하십시오.)
계산 결과:
작업 농도: mg/ml;
DMSO 원액 준비 방법: mg 약물 사전 용해 μL DMSO ( 원액 농도 mg/mL, 농도가 해당 약물 배치의 DMSO 용해도를 초과하는 경우 먼저 당사에 문의하십시오. )
생체 내 제형 준비 방법: 취하다 μL DMSO 원액, 다음 추가μL PEG300, 혼합하고 투명하게 한 다음 추가μL Tween 80, 혼합하고 투명하게 한 다음 추가 μL ddH2O, 혼합하고 투명하게 합니다.
생체 내 제형 준비 방법: 취하다 μL DMSO 원액, 다음 추가 μL 옥수수 기름, 혼합하고 투명하게 합니다.
참고: 1. 다음 용매를 추가하기 전에 액체가 투명한지 확인하십시오.
2. 용매를 순서대로 추가해야 합니다. 다음 용매를 추가하기 전에 이전 추가에서 얻은 용액이 투명한 용액인지 확인해야 합니다. 와동, 초음파 또는 뜨거운 물 중탕과 같은 물리적 방법을 사용하여 용해를 도울 수 있습니다.
작용 메커니즘
| Targets/IC50/Ki |
FIPV
(Cell-free assay) 0.78 μM(EC50)
|
참조 |
|---|
임상시험 정보
(데이터 출처 https://clinicaltrials.gov, 업데이트 날짜 2024-05-22)
| NCT 번호 | 모집 | 조건 | 스폰서/협력자 | 시작일 | 단계 |
|---|---|---|---|---|---|
| NCT05996744 | Terminated | COVID-19 |
Gilead Sciences |
December 26 2023 | Phase 2|Phase 3 |
| NCT05715528 | Completed | COVID-19 |
Gilead Sciences |
February 8 2023 | Phase 3 |
| NCT05603143 | Terminated | COVID-19 |
Gilead Sciences |
November 5 2022 | Phase 3 |
| NCT04582266 | Completed | COVID-19 |
National Institute of Allergy and Infectious Diseases (NIAID)|Gilead Sciences |
March 31 2021 | -- |
| NCT04859244 | Completed | COVID-19 |
Copycat Sciences LLC |
January 1 2021 | Phase 1 |
| NCT04539262 | Completed | COVID-19 |
Gilead Sciences |
September 14 2020 | Phase 1|Phase 2 |
