Wnt proteins form a family of highly conserved secreted signaling molecules that regulate cell-to-cell interactions during embryogenesis. Wnt signalling pathway plays a key role in the development of CSC(Cancer stem cell) and it may be a significant step in the development of a large number of tomours. As currently understood, Wnt proteins bind to receptors of the Frizzled and LRP families on the cell surface. [show the full text]
Wnt/beta-catenin 억제제/활성제 (Wnt/beta-catenin Inhibitors/Activators)
기타 Stem Cells & Wnt 억제제
| Cat.No. | 제품명 | 정보 | 제품 사용 인용 | 제품 검증 |
|---|---|---|---|---|
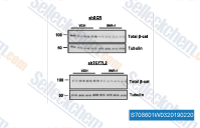
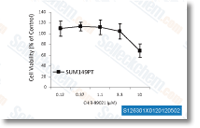
| S7086 | IWR-1-endo | IWR-1-endo (endo-IWR 1, IWR-1)는 Wnt3A를 발현하는 L-세포에서 IC50이 180 nM인 Wnt 경로 억제제로, Axin2 단백질 수치를 유도하고 Axin 스캐폴드 파괴 복합체를 안정화하여 β-카테닌 인산화를 촉진합니다. |
|
|
| S8968 | PRI-724 (Foscenvivint) | Foscenvivint (PRI-724)는 β-카테닌과 CBP의 상호작용을 방해하는 강력하고 특이적인 억제제입니다. |
|
|
| S7085 | IWP-2 | IWP-2는 무세포 분석에서 IC50이 27 nM인 Wnt 처리 및 분비 억제제이며, Porcn 매개 Wnt 팔미토일화를 선택적으로 차단하고, 일반적으로 Wnt/β-catenin에 영향을 미치지 않으며, Wnt 자극 세포 반응에 대한 효과를 나타내지 않습니다. IWP-2는 특히 CK1δ를 억제합니다. |
|
|
| S0733 | Tegatrabetan (BC-2059) | β-카테닌의 저해제인 Tegatrabetan (BC-2059, Tegavivint)은 β-카테닌과 스캐폴드 단백질 트랜스듀신 β-like 1 (TBL1)의 결합 및 프로테아좀 분해를 방해하여 β-카테닌의 핵 수준을 감소시킵니다. |
|
|
| S3842 | Isoquercitrin | Bidens bipinnata L에서 분리된 항암 활성을 가진 플라보노이드 화합물인 Isoquercitrin (Hirsutrin, 3-Glucosylquercetin, Quercetin 3-o-glucopyranoside)은 A-카테닌 핵 전위의 하류에서 작용하는 Wnt/ A-카테닌의 억제제입니다. |
|
|
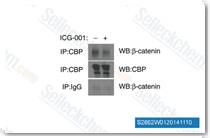
| S2662 | ICG-001 | ICG-001은 Wnt/β-catenin/TCF 매개 전사를 길항하고 CREB 결합 단백질(CBP)에 특이적으로 결합하며 IC50은 3 μM이지만, 관련 전사 보조 활성인자 p300은 아닙니다. ICG-001은 세포자멸사를 유도합니다. |
|
|
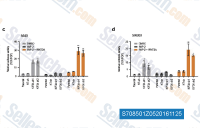
| S1263 | CHIR-99021 (Laduviglusib) | Laduviglusib (CHIR-99021, CT99021)는 IC50 값이 각각 10 nM 및 6.7 nM인 GSK-3α 및 GSK-3β 억제제입니다. 이는 사이클린 의존성 키나아제(CDKs)에 대한 교차 반응성을 나타내지 않으며 CDKs에 비해 GSK-3β에 대해 350배의 선택성을 보입니다. 이 화합물은 Wnt/β-catenin 활성제로 기능하며 autophagy를 유도합니다. |
|
|
| S2924 | Laduviglusib (CHIR-99021) Hydrochloride | Laduviglusib (CHIR-99021; CT99021) HCl은 CHIR-99021의 염산염으로, IC50이 10 nM/6.7 nM인 GSK-3α/β 억제제입니다. CHIR-99021은 가장 가까운 동족체인 Cdc2 및 ERK2에 비해 GSK-3에 대해 500배 이상의 선택성을 보입니다. CHIR-99021은 Wnt/beta-catenin 신호 전달 경로의 강력한 약리학적 활성제입니다. CHIR-99021은 빛 유도 autophagy를 유의하게 구조하고 GR, RORα 및 autophagy 관련 단백질을 증가시킵니다. |
|
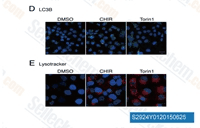
|
| S1180 | XAV-939 | XAV-939 (NVP-XAV939)는 세포 없는 분석에서 11 nM/4 nM의 IC50으로 tankyrase1/2 억제를 통해 Wnt/β-catenin 매개 전사를 선택적으로 억제하고, 액신 수치를 조절하며 CRE, NF-κB 또는 TGF-β에 영향을 미치지 않습니다. |
|
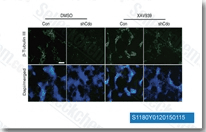
|
| S5992 | Heparan Sulfate | Heparan sulfate (HS, Heparitin sulfate, Alpha-idosane, HHS 5, N-Acetylheparan Sulfate, Suleparoid, Tavidan)는 HS 프로테오글리칸(HSPG)의 구성 요소이며, 세포 표면에 존재하는 선형 다당류입니다. Heparan sulfate는 장 상피 세포(IEC)의 Wnt에 대한 결합 친화도에 영향을 미쳐 정규 Wnt 신호 전달의 활성화를 촉진하고 상피 손상 후 소장 크립트의 재생을 촉진합니다. |
|
|
The Wnt/β-catenin signalling pathway is an important mechanism of study for researchers of human diseases as it plays a role in oncology when overactivated, however, reduced signalling of this pathway also results in abnormal bone development and neurodegenerative illnesses. Depending on the indication of use, development of inhibitors or activators of the Wnt/β-catenin signalling pathway is an important area of focus.
Normally, regulation of the Wnt/β-catenin signalling pathway occurs by a large protein assembly called the β-catenin destruction complex that maintains low concentrations of β-catenin in the cytoplasm, and consequently in the nucleus. The β-catenin destruction complex is comprised of glycogen synthase kinase 3 (GSK3α/ GSK3β), casein kinase Iα (CKIα), Axin1/Axin2 scaffolding, and adenomatous polyposis coli (APC) – a tumor suppressor protein. Recruitment of β-catenin to the destruction complex leads to the phosphorylation of β-catenin N-terminus residues by CKIα and GSK3, following which ubiquitination and degradation events occur. Sequestering β-catenin at several N-terminal serine and threonine residues to the β-catenin destruction complex are Axin and APC. Similarly, it is believed these constituents of the β-catenin destruction complex regulate β-catenin efflux from the nucleus. The low concentration of β-catenin in the cell restricts β-catenin to the essential role of cadherin-mediated cell adhesion. An additional mechanism controlling the expression of Wnt signalling in the cell involves the inhibition of Wnt specific gene transcription by the T-cell factor (TCF) family of proteins.[1]
Activation of the Wnt/β-catenin signalling pathway is signified by the formation of a “Wnt signalosome” – a large protein complex that develops following the recognition of Wnt protein on the cell surface by a set of proteins called the seven-pass transmembrane Frizzled (Fz) family and its co-receptor, low density lipoprotein receptor related protein (LRP5/LRP6).ii The Wnt signalosome inhibits the activity of the β-catenin destruction complex by recruiting several components of the destruction complex to the membrane. As a consequence, the buildup of β-catenin’s unphosphorylated form results in the cytoplasm and subsequently in the nucleus. It is in the nucleus where rising concentrations of β-catenin combine with TCF proteins to transform this protein from an inhibitory protein to an activator of Wnt-signalling pathway by encouraging the transcription of the Wnt-responsive gene.[1]
Among cancer research, Wnt/β-catenin signalling is an area of focus since loss-of-function mutations in the APC gene results in β-catenin stabilization. Deletions within a chromosomal region containing the APC gene is understood to be associated with a hereditary disease known as familial adenomatous polyposis that yield intestinal polyps in large numbers that ultimately gives rise to tumors. Alternatively, mutations that promote gain-of-function of the APC gene inhibit the β-catenin destruction complex from limiting β-catenin concentrations in the cell. Additionally, some cancers have been correlated to a loss of Axin1 or Axin2 function. Overexpression of the Wnt/ β-catenin signalling pathway results in the constitutive activation of c-myc, and is most commonly linked to colorectal cancer.[2]
Since the Wnt/β-catenin signalling pathway plays a critical role in cancer, clinical research has yielded several new targets that may positively influence Wnt/β-catenin signalling where its absence is related to disease. Potential new drug targets include two lipid kinases that were found with the accumulation of β-catenin among HEK293T cells transfected with short inhibitory RNAs (siRNAs) targeting human kinases, these include: (1) phosphatidylinositol 4-kinase type IIα (PI4KIIα), and (2) phosphatidylinositol-4-phosphate 5-kinase type Iβ (PIP5Kiβ). Additionally, three human homologs of Drosophila PAR-1 can also provide useful drug targets as these elements activate Wnt/β-catenin signalling pathway as well.[2]
There are many enzymes involved in the Wnt/β-catenin signalling pathway and knowing whether activation or repression is best suited will determine the molecular strategy to explore for therapeutic benefit.
