NF-κB controls the transcription of DNA. NF-κB is found in almost all animal cell types and is involved in cellular responses to stimuli such as stress, cytokines, free radicals, ultraviolet irradiation, oxidized LDL, and bacterial or viral antigens. [show the full text]
NF-κB 억제제 (NF-κB Inhibitors)
| Cat.No. | 제품명 | 정보 | 제품 사용 인용 | 제품 검증 |
|---|---|---|---|---|
| E4686 | DCZ0415 | DCZ0415는 TRIP13의 강력한 억제제입니다. DCZ0415는 비상동 말단 연결 복구를 손상시키고 NF-κB 활성을 억제합니다. 이는 시험관 내외에서, 그리고 약물에 내성이 있는 다발성 골수종 환자로부터 얻은 1차 세포에서 항골수종 효과를 유발합니다. |
|
|
| S7672 | Omaveloxolone (RTA-408) | Omaveloxolone (RTA-408)은 세포 보호 전사 인자 Nrf2를 활성화하고 NF-κB 신호 전달을 억제하는 합성 트리테르페노이드입니다. 2상. |
|
|
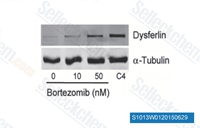
| S1013 | Bortezomib | Bortezomib은 Ki가 0.6 nM인 강력한 20S proteasome 억제제입니다. 이는 정상 세포보다 종양 세포에 대해 유리한 선택성을 나타냅니다. 이 화합물은 NF-κB를 억제하고 ERK 인산화를 유도하여 Cathepsin B를 억제하고 난소암 및 기타 고형암에서 autophagy의 촉매 과정을 억제합니다. |
|
|
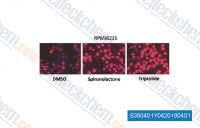
| S3604 | Triptolide | Triptolide는 중국 약초 Tripterygium wilfordii에서 추출한 디테르펜 트리에폭사이드 면역억제제입니다. 이는 p65/CBP 상호작용 방해 및 p65 단백질 감소를 통해 이중 작용으로 NF-κB 억제제 역할을 합니다. Triptolide (PG490)는 열충격 전사 인자 1 (HSF1)의 전사 활성 기능을 제거합니다. Triptolide는 MDM2를 억제하고 p53 비의존적 경로를 통해 세포자멸사를 유도합니다. |
|
|
| S8341 | TAK-243 (MLN7243) | TAK-243 (MLN7243)은 UBCH10 E2 티오에스테르 분석에서 IC50이 1 ± 0.2 nM인 ubiquitin activating enzyme (UAE)의 강력한 기전 기반 소분자 억제제입니다. 이는 다양한 키나아제 및 수용체 분석뿐만 아니라 인간 탄산 무수화 효소 1형 및 2형에 대해 최소한의 억제 활성을 보입니다. TAK-243 (MLN7243)은 ER stress를 유도하고, NF-κB 경로 활성화를 차단하며 apoptosis를 촉진합니다. |
|
|
| S8483 | CBL0137 Hydrochloride | CBL0137 (CBLC137, Curaxin 137) HCl은 세포 기반 p53 및 NF-kB 리포터 분석에서 각각 0.37 μM 및 0.47 μM의 EC50으로 p53을 활성화하고 NF-κB를 억제합니다. 또한 히스톤 샤페론 FACT (facilitates chromatin transcription complex)를 억제합니다. |
|
|
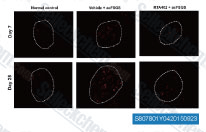
| S8078 | Bardoxolone Methyl (RTA 402) | Bardoxolone Methyl (RTA 402, TP-155, NSC 713200, CDDO Methyl Ester, CDDO-Me)은 강력한 세포자멸사 촉진 및 항염증 활성을 나타내는 IKK 억제제이며, 강력한 Nrf2 활성제이자 핵인자-κB (NF-κB) 억제제입니다. Bardoxolone Methyl은 페로프토시스를 억제합니다. Bardoxolone Methyl은 암세포에서 세포자멸사 및 자가포식을 유도합니다. |
|
|
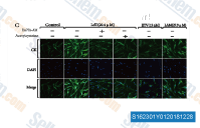
| S1623 | N-Acetylcysteine (NAC chemical, N-Acetyl-L-Cysteine) | Acetylcysteine (N-acetyl-l-cysteine, NAC, N-acetylcysteine)은 프로테아좀 억제제의 활동을 저해하는 ROS(활성산소종) 억제제입니다. 또한 종양 괴사 인자 생성 억제제이기도 합니다. Acetylcysteine (N-acetyl-l-cysteine)은 IκB 키나아제의 억제를 통해 TNF 유도 NF-κB 활성화를 억제합니다. Acetylcysteine (N-acetyl-l-cysteine)은 미토콘드리아 의존 경로를 통해 apoptosis를 유도합니다. Acetylcysteine (N-acetyl-l-cysteine)은 ferroptosis와 바이러스 복제를 억제합니다.용액은 불안정하므로 신선하게 준비해야 합니다. |
|
|
| S2913 | BAY 11-7082 (BAY 11-7821) | BAY 11-7082 (BAY 11-7821)는 NF-κB 억제제로, 종양 세포에서 TNFα 유도 IκBα 인산화를 IC50 10 μM로 억제합니다. BAY 11-7082는 유비퀴틴 특이적 프로테아제 USP7 및 USP21을 각각 IC50 0.19 μM 및 0.96 μM로 억제합니다. BAY 11-7082는 위암 세포에서 아폽토시스 및 S기 정지를 유도합니다. |
|
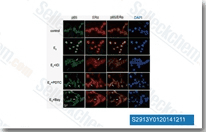
|
| S7351 | JSH-23 | JSH-23은 NF-κB 전사 활성 억제제로, RAW 264.7 세포에서 7.1 μM의 IC50 값으로 LPS-자극 핵인자 (NF)-κB 전사 활성을 억제하고, IκB 분해에 영향을 미치지 않으면서 LPS 유도 NF-κB 핵 전위에 간섭합니다. |
|

|
NF-κB (nuclear factor-kappa B) is a highly regulated, homo- or hetero-dimeric transcription factor, present in almost all cell types. The NF-κB proteins are composed of five different subunits, RelA (p65), RelB, c-Rel (Rel), NF-κB1, and NF-κB2, all of which share a Rel homology domain (RHD) in their N-termini, and have a transactivation domain in their C-termini, except for NF-κB1 and NF-κB2. The NF-κB1 and NF-κB2 proteins are synthesized as longer precursors, p105, and p100, which undergo selective degradation of their C-terminal region containing ankyrin repeats to generate the active NF-κB subunits, p50 and p52, respectively. [i] Different dimer combinations act as transcriptional activators or repressors, respectively. The p50 and p52 NF-κB members play critical roles in modulating the specificity of NF-κB function by forming heterodimers with RelA, RelB, or c-Rel. The NF-κB RelA-p50 and RelB-p50 heterodimeric complexes are transcriptional activators. The NF-κB p50/p50 and p52/p52 homodimers are generally transcriptional repressors, but can function as transcriptional activators when bound to nuclear protein Bcl-3. [2]
NF-κB is a rapidly acting primary transcription factor, and is controlled by subcellular compartmentalization and post-translational modifications (PTMs) including phosphorylation, acetylation, methylation and ubiquitylation. NF-κB dimers are primarily sequestered as an inactive form in the cytoplasm by a protein complex called inhibitor of kappa B (IκB) among unstimulated cells. Activation of NF-κB occurs via the degradation of IκB, a process initiated by IκB kinase (IKK). A variety of stimuli such as cytokines and cellular stress can activate the IKK, resulting in ubiquitination and dissociation of the IκB from NF-κB. The activated NF-κB is then translocated into the nucleus to regulate gene expression. NF-κB regulates a broad range of genes involved in various biological processes including inflammation, immunity, differentiation, development, as well as genes regulating cell proliferation, apoptosis, cell adhesion and the cellular microenviroment. In addition, NF-κB activates its own repressor IκBα and IκBε, as well as the TNFAIP3 (A20) a negative regulator of IKK activation, forming a negative feedback loop. [1]
NF-κB has been found to be constitutively active in a number of diseases, including arthritis, chronic inflammation, asthma, neurodegenerative diseases, and heart disease, as well as in many types of human tumors. [ii] NF-κB has long been linked with cancer, primarily through aberrant constitutive NF-κB activation that suppresses apoptosis or promotes tumor growth, metastasis, and angiogenesis by inducing the expression of anti-apoptotic genes, proto-oncogenes, matrix metalloproteinase, cell adhesion genes, and genes associated with the growth of new blood vessels. Additionally, NF-κB promotes a metabolic switch in cancer cells from oxidative phosphorylation to glycolysis (the Warburg effect) by inducing the expression of glycolytic enzymes and inhibiting the expression of mitochondrial gene. Constitutive activation of NF-κB can result from continuous exposure to NF-κB activating stimuli, such as cytokine release by tumor-associated macrophages (TAMs), or from mutations in NF-κB subunits and genes involved in regulating NF-κB function. Inhibiting NF-κB activation can prevent tumor cell proliferation and induce cell death. Given the importance of NF-κB in initiating or enhancing cell survival, NF-κB is therefore considered as a promising target for anticancer therapies. [1]
