연구용
Doxycycline MMP Inhibitor
제품 번호S5159
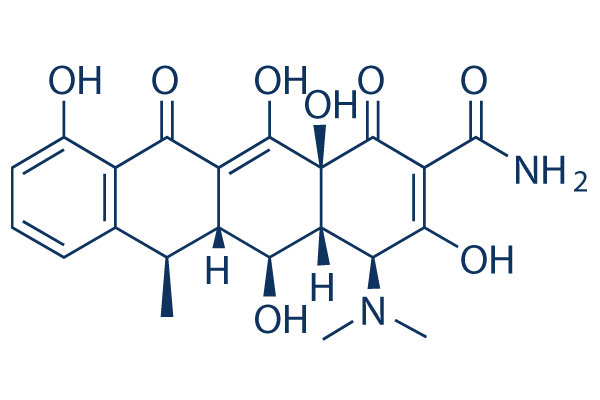
화학 구조
분자량: 444.43
품질 관리
| 관련 타겟 | Integrase Bacterial Antibiotics Anti-infection Fungal Antiviral COVID-19 Parasite Reverse Transcriptase HIV |
|---|---|
| 기타 Antineoplastic and Immunosuppressive Antibiotics 억제제 | Staurosporine (STS) Cyclosporin A Oligomycin A (MCH 32) Puromycin Dihydrochloride Nigericin sodium salt Geldanamycin (NSC 122750) Honokiol Streptozotocin (STZ) Sodium Monensin (NSC 343257) Cephalomannine |
세포 배양, 처리 및 작업 농도
| 세포주 | 분석 유형 | 농도 | 배양 시간 | 제형 | 활성 설명 | PMID |
|---|---|---|---|---|---|---|
| Staphylococcus aureus 2 planktonic cells | Bactericidal assay | 24 hrs | Bactericidal activity against methicillin-resistant Staphylococcus aureus 2 planktonic cells after 24 hrs by calgary biofilm device method, MBC=2μM. | 29638121 | ||
| THP1 | Function assay | Selectivity index, ratio of IC50 for human THP1 cells to IC50 for Plasmodium falciparum, IC50=3.1μM. | 19748781 | |||
| K562 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human K562 cells after 72 hrs by flow cytometry, IC50=15μM. | 19482476 | ||
| K562 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human K562 cells after 72 hrs by flow cytometry, IC50=15μM. | 19926173 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells after 72 hrs by MTT assay, IC50=20μM. | 19482476 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells after 72 hrs by MTT assay, IC50=20μM. | 19926173 | ||
| THP1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human THP1 cells after 72 hrs by propidium iodide staining-based flow cytometry, IC50=20μM. | 19748781 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells after 72 hrs by MTT assay, CC50=20μM. | 21741131 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells after 72 hrs by MTT assay, CC50=20μM. | 22889559 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells after 72 hrs by MTT assay, CC50=20μM. | 21852132 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells after 72 hrs by MTT assay, CC50=20μM. | 24946216 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells assessed as cell viability after 72 hrs by MTT assay, CC50=20μM. | 25791675 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells incubated for 72 hrs by MTT assay, CC50=20μM. | 25282267 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells measured after 72 hrs by MTT assay, CC50=20μM. | 27155463 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells assessed as reduction in cell viability after 72 hrs by MTT assay, CC50=20μM. | 27654395 | ||
| SW1353 | Function assay | 50 uM | Inhibition of IL-1-beta-induced MMP13 production in human SW1353 cells at 50 uM | 17267227 | ||
| K-12 BW25113 | Bactericidal assay | 24 hrs | Bactericidal activity against yafQ gene-deficient Escherichia coli K-12 BW25113 biofilm assessed as log reduction of viable cells after 24 hrs | 19307375 | ||
| K-12 BW25113 | Bactericidal assay | 24 hrs | Bactericidal activity against Escherichia coli K-12 BW25113 biofilm harboring pCA24N ptac::yafQ plasmid assessed as log reduction of viable cells after 24 hrs pretreated with 5 uM of IPTG for 4 hrs | 19307375 | ||
| Neuro2a | Function assay | 1 uM | Decrease in 7-DHC levels in Dhcr7-deficient mouse Neuro2a cells at 1 uM by LC-MS/GC-MS analysis | 26789657 | ||
| vascular endothelial cells | Antibacterial assay | 25 ug/ml | 72 hrs | Antibacterial activity against Rickettsia prowazekii str. Breinl infected in CD rat primary pulmonary vascular endothelial cells assessed as bacterial shape change at 25 ug/ml measured 72 hrs post infection by Hoechst 33258/phalloidin staining based fluor | 28089350 | |
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | |||
| BT-12 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-12 cells | 29435139 | |||
| Hep2 | Anti-Chlamydial assay | 1 ug/ml | 8 hrs | Anti-Chlamydial activity against Chlamydia trachomatis Serovar LGV-L2 infected in Hep2 cells assessed as reduction in size and number of chlamydial inclusion at 1 ug/ml treated at 8 hrs post-infection and 24 hrs later re-infecting fresh Hep2 cells monolay | 32227948 | |
| skeletal myoblast cells | Cytotoxicity assay | DNDI: Cytotoxicity in Vitro, 72 hour, in rat skeletal myoblast cells, IC50=14.49μM. | ChEMBL | |||
| 클릭하여 더 많은 세포주 실험 데이터 보기 | ||||||
화학 정보, 보관 및 안정성
| 분자량 | 444.43 | 화학식 | C22H24N2O8 |
보관 (수령일로부터) | |
|---|---|---|---|---|---|
| CAS 번호 | 564-25-0 | SDF 다운로드 | 원액 보관 |
|
|
| 동의어 | Vibramycin, Doxytetracycline, Doxiciclina, Doxycyclinum | Smiles | CC1C2C(C3C(C(=O)C(=C(C3(C(=O)C2=C(C4=C1C=CC=C4O)O)O)O)C(=O)N)N(C)C)O | ||
용해도
|
In vitro |
DMSO
: 89 mg/mL
(200.25 mM)
Water : Insoluble Ethanol : Insoluble |
몰농도 계산기
|
In vivo |
|||||
생체 내 제형 계산기 (투명한 용액)
1단계: 아래 정보 입력 (권장: 실험 중 손실을 고려하여 추가 동물 포함)
2단계: 생체 내 제형 입력 (이것은 계산기일 뿐 제형이 아닙니다. 용해도 섹션에 생체 내 제형이 없는 경우 먼저 당사에 문의하십시오.)
계산 결과:
작업 농도: mg/ml;
DMSO 원액 준비 방법: mg 약물 사전 용해 μL DMSO ( 원액 농도 mg/mL, 농도가 해당 약물 배치의 DMSO 용해도를 초과하는 경우 먼저 당사에 문의하십시오. )
생체 내 제형 준비 방법: 취하다 μL DMSO 원액, 다음 추가μL PEG300, 혼합하고 투명하게 한 다음 추가μL Tween 80, 혼합하고 투명하게 한 다음 추가 μL ddH2O, 혼합하고 투명하게 합니다.
생체 내 제형 준비 방법: 취하다 μL DMSO 원액, 다음 추가 μL 옥수수 기름, 혼합하고 투명하게 합니다.
참고: 1. 다음 용매를 추가하기 전에 액체가 투명한지 확인하십시오.
2. 용매를 순서대로 추가해야 합니다. 다음 용매를 추가하기 전에 이전 추가에서 얻은 용액이 투명한 용액인지 확인해야 합니다. 와동, 초음파 또는 뜨거운 물 중탕과 같은 물리적 방법을 사용하여 용해를 도울 수 있습니다.
작용 메커니즘
| 시험관 내(In vitro) |
100 ng/mL-5 µg/mL Doxycycline은 세포의 대사 프로필을 유의하게 변경하고 증식 속도를 감소시킬 수 있지만, 효과의 크기는 사용된 특정 세포주에 따라 달라집니다. 이 화합물은 PANC-1 세포에서 세포 주기의 G1-S기에서 세포를 정지시키고 p53 및 그 하류 표적 p21의 발현을 유도합니다. 항세포자멸 유전자를 하향 조절하고 전세포자멸 유전자를 유도합니다. 이 화학물질은 또한 Jurkat T 림프구에서 Fas/Fas-리간드 의존성 경로를 통해 세포자멸사를 유도합니다. |
|---|---|
| 생체 내(In vivo) |
Doxycycline은 생체 내에서 종양 성장을 성공적으로 감소시켰습니다 (췌장암 세포 성장 억제). |
참조 |
|
적용 분야
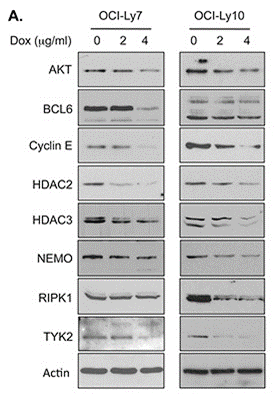
| 방법 | 바이오마커 | 이미지 | PMID |
|---|---|---|---|
| Western blot | AKT / BCL6 / Cyclin E / HDAC2 / HDAC3 / NEMO / TYK2 / RIPK1 HSP70 / HSP90 pATM / ATM |
|
26142707 |
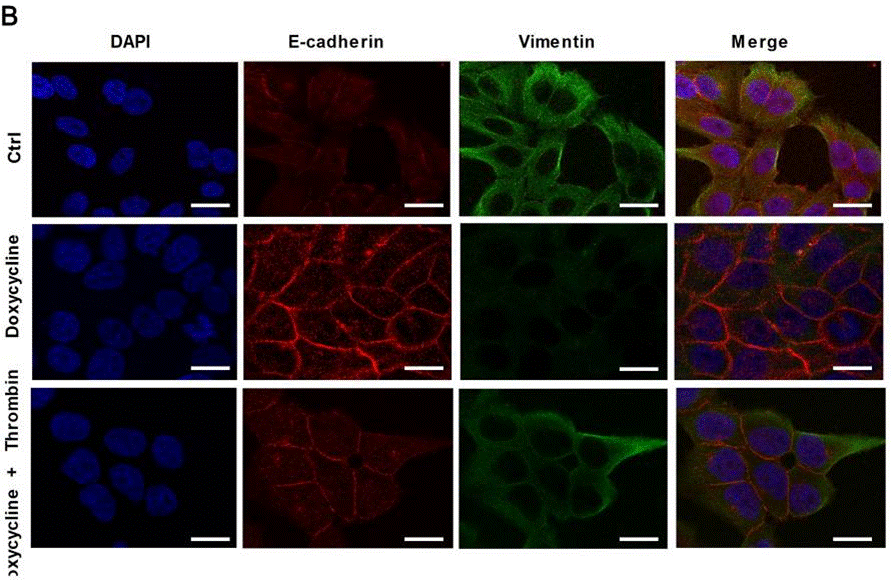
| Immunofluorescence | E-cadherin / Vimentin |
|
29285218 |
임상시험 정보
(데이터 출처 https://clinicaltrials.gov, 업데이트 날짜 2024-05-22)
| NCT 번호 | 모집 | 조건 | 스폰서/협력자 | 시작일 | 단계 |
|---|---|---|---|---|---|
| NCT05972772 | Not yet recruiting | Infectious Disease|Therapeutics |
Lao-Oxford-Mahosot Hospital Wellcome Trust Research Unit|Mahidol Oxford Tropical Medicine Research Unit |
March 20 2024 | Phase 2|Phase 3 |
| NCT06007534 | Recruiting | Post-exposure Prophylaxis|Sexually Transmitted Diseases|Doxycycline |
Assistance Publique - Hôpitaux de Paris |
October 25 2023 | Not Applicable |
| NCT04762134 | Recruiting | Bacterial Sexually Transmitted Diseases |
Jonathan Troy Grennan|Canadian Institutes of Health Research (CIHR)|British Columbia Centre for Disease Control |
June 2 2023 | Phase 4 |
| NCT05853120 | Recruiting | Sexually Transmitted Diseases |
Emory University|Centers for Disease Control and Prevention |
May 31 2023 | Phase 4 |
| NCT05382208 | Recruiting | Emphysema|HIV |
Weill Medical College of Cornell University|National Heart Lung and Blood Institute (NHLBI)|University of California Los Angeles|University of Iowa|University of Michigan |
August 22 2022 | Phase 2 |
| NCT05492019 | Recruiting | Parkinson Disease |
Bangabandhu Sheikh Mujib Medical University Dhaka Bangladesh |
July 1 2022 | Phase 2 |
