연구용
Lamivudine (3TC, BCH-189) Reverse Transcriptase 억제제
제품 번호S1706
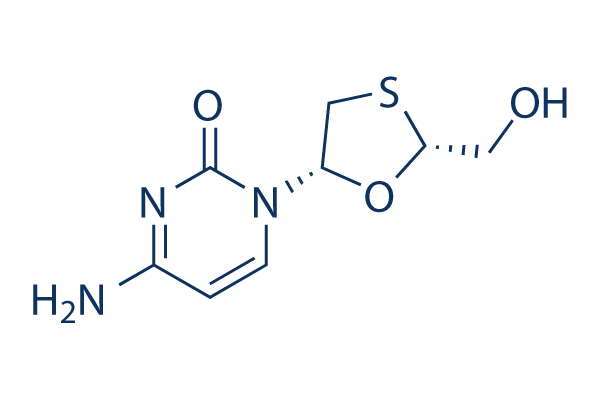
화학 구조
분자량: 229.26
품질 관리
세포 배양, 처리 및 작업 농도
| 세포주 | 분석 유형 | 농도 | 배양 시간 | 제형 | 활성 설명 | PMID |
|---|---|---|---|---|---|---|
| MT-4 | Function assay | Concentration required to inhibit syntica formation by 50% in HIV-1 infected MT-4 cells, IC50=0.1μM | 8035429 | |||
| PBMC | Function assay | Concentration required for antiviral activity in PBMC (human peripheral blood mononuclear cells) infected with HIV-1 strain LAV, EC50=0.0018μM | 8410975 | |||
| PBMC | Function assay | Concentration required for antiviral activity in PBMC (human peripheral blood mononuclear cells) infected with HIV-1 strain LAV, EC50=0.21μM | 8410975 | |||
| PBMC | Anti-HIV-1 assay | Anti-HIV-1 activity against human peripheral blood mononuclear (PBM) cells infected with HIV-1 strain LAV, EC50=0.0018μM | 8423591 | |||
| PBMC | Anti-HIV-1 assay | Anti-HIV-1 activity against human peripheral blood mononuclear (PBM) cells infected with HIV-1 strain LAV, EC50=0.06μM | 8423591 | |||
| PBMC | Anti-HIV-1 assay | Anti-HIV-1 activity against human peripheral blood mononuclear (PBM) cells infected with HIV-1 strain LAV, EC50=0.21μM | 8423591 | |||
| PBMC | Anti-HIV-1 assay | Anti-HIV-1 activity against human peripheral blood mononuclear (PBM) cells infected with HIV-1 strain LAV, EC50=10.1μM | 8423591 | |||
| 2.2.15 | Function assay | In vitro effective concentration against hepatitis B virus was determined in 2.2.15 cells, EC50=0.008μM | 9301659 | |||
| PBMC | Anti-HIV-1 assay | In vitro anti-HIV activity in peripheral blood mononuclear cells, EC50=0.07μM | 9301659 | |||
| 2.2.15 | Antiviral assay | Antiviral activity against Hepatitis B virus (HBV) in 2.2.15 cells, EC50=0.008μM | 11052795 | |||
| PBMC | Antiviral assay | Antiviral activity against Immunodeficiency virus type-1 (HIV-1) in peripheral blood mononuclear cells, EC50=0.07μM | 11052795 | |||
| H9 | Antiviral assay | Antiviral activity against HIV1 3B in human H9 cells assessed as inhibition of virus-induced cytopathic effect by formazan-based conventional colorimetric technique, EC50=0.06μM | 11430019 | |||
| H9 | Antiviral assay | Antiviral activity against HIV1 RF in human H9 cells assessed as inhibition of virus-induced cytopathic effect by formazan-based conventional colorimetric technique, EC50=0.06μM | 11430019 | |||
| PBMC | Antiviral assay | Antiviral activity against HIV-1xxBRU mutant strain in human PBM cells, EC90=0.08μM | 12383014 | |||
| PBMC | Function assay | Activity against Lamivudine-resistant virus (HIV-1XXBRU) in human PBM cells, expressed as EC50, EC50=0.035μM | 12852755 | |||
| PBMC | Function assay | In vitro concentration required to inhibit the HIV-1 LA1 replication on PBMCs cell (peripheral blood mononuclear cells), EC50=0.01μM | 12852943 | |||
| PBMC | Function assay | Effective concentration to inhibit HIV-1 LAI cytopathicity in PBMC cells was determined in vitro, EC50=0.0116μM | 14971898 | |||
| HepG2.2.15 | Function assay | Effective concentration to inhibit hepatitis B virus cytopathicity in HepG2.2.15 cells was determined in vitro, EC50=0.2μM | 14971898 | |||
| MT-4 | Function assay | Effective concentration to inhibit HIV-1 IIIB cytopathicity in MT-4 cells was determined in vitro, EC50=0.5μM | 14971898 | |||
| PBMC | Antiviral assay | Antiviral activity against wild type HIV virus(xxBRU) in Human peripheral blood mononuclear (PBM) cells, EC90=0.08μM | 15027854 | |||
| PBMC | Function assay | Effective concentration against Wild Type HIV-1 virus in human peripheral blood mononuclear cells, EC50=0.041μM | 15189036 | |||
| CEM-SS | Antiviral assay | 6 days | Antiviral activity against HIV1 3B infected in human CEM-SS cells assessed as inhibition of virus-induced cytopathic effect after 6 days by MTS assay, ED50=0.36μM | 15217281 | ||
| HOG.R5 | Antiviral assay | 4 days | Antiviral activity against HIV1 3B infected in human HOG.R5 cells after 4 days, IC50=1.2μM | 15217281 | ||
| 2.2.15 | Function assay | Inhibition of HBV replication in 2.2.15 cells by DNA hybridization assay, EC50=0.02μM | 15615545 | |||
| 2.2.15 | Function assay | Effective concentration required to inhibit hepatitis B virus replication in 2.2.15 cells in DNA hybridization assay, EC50=0.05μM | 15634003 | |||
| PBMC | Function assay | In vitro effective concentration against Lamivudine resistant wild type HIV-1 in human peripheral blood mononuclear cells, EC50=0.027μM | 15916425 | |||
| PBMC | Function assay | In vitro effective concentration against Lamivudine resistant wild type HIV-1 in human peripheral blood mononuclear cells, EC90=0.25μM | 15916425 | |||
| 2.2.15 | Function assay | Effective concentrations at which 2-fold depression of extracellular virion DNA (HBV) was observed in 2.2.15 Cells, EC50=0.06μM | 16033283 | |||
| 2.2.15 | Function assay | Effective concentrations at which 2-fold depression of extracellular virion DNA (HBV) was observed in 2.2.15 Cells, EC90=0.17μM | 16033283 | |||
| 2.2.15 | Function assay | Effective concentrations at which 10-fold depression of intracellular intermediates of HBV DNA was observed in 2.2.15 Cells, EC50=0.23μM | 16033283 | |||
| 2.2.15 | Function assay | Effective concentrations at which 10-fold depression of intracellular intermediates of HBV DNA was observed in 2.2.15 Cells, EC90=0.69μM | 16033283 | |||
| 2.2.15 | Function assay | Inhibition of synthesis of extracellular virion DNA release in cultured human hepatoblastoma 2.2.15 cells, EC50=0.048μM | 16213713 | |||
| 2.2.15 | Function assay | Inhibition of synthesis of extracellular virion DNA release in cultured human hepatoblastoma 2.2.15 cells, EC90=0.151μM | 16213713 | |||
| 2.2.15 | Function assay | Inhibition of synthesis of intracellular HBV DNA replicative intermediates in cultured human hepatoblastoma 2.2.15 cells, EC50=0.251μM | 16213713 | |||
| 2.2.15 | Function assay | Inhibition of synthesis of intracellular HBV DNA replicative intermediates in cultured human hepatoblastoma 2.2.15 cells, EC90=0.648μM | 16213713 | |||
| 2.2.15 | Function assay | Inhibition of wild type HBV transfected in human hepatoblastoma 2.2.15 cells, EC50=2.1μM | 16539393 | |||
| hepatoma 2.2.15 | Antiviral assay | Antiviral activity against wild type HBV in human hepatoma 2.2.15 cells, EC50=2.1μM | 16759112 | |||
| 2.2.15 | Function assay | Inhibition of HBV replication in 2.2.15 cells by DNA hybridization assay, EC50=0.02μM | 17004726 | |||
| MDCK2 | Function assay | 10 uM | Inhibition of human MRP2 expressed in MDCK2 cells assessed as increase in intracellular CMF fluorescence at 10 uM by CMFDA assay | 17172311 | ||
| MDCK2 | Function assay | 10 uM | Inhibition of human MRP3 expressed in MDCK2 cells assessed as increase in intracellular CMF fluorescence at 10 uM by CMFDA assay | 17172311 | ||
| MDCK2 | Function assay | 10 uM | Inhibition of human MRP1 expressed in MDCK2 cells assessed as increase in intracellular CMF fluorescence at 10 uM by CMFDA assay | 17172311 | ||
| Huh7 | Function assay | Inhibition of wild type HBV replication in Huh7 cells, EC50=1μM | 17371827 | |||
| PBMC | Antiviral assay | Antiviral activity against HIV1 LAI in human PBM cells, EC50=0.026μM | 17403996 | |||
| PBMC | Antiviral assay | Antiviral activity against HIV1 LAI in human PBM cells, EC90=0.12μM | 17403996 | |||
| HepG2.2.15 | Antiviral assay | Antiviral activity against HBV in HepG2.2.15 cells assessed as decrease in extracellular viral DNA level by RT-PCR, EC50=0.1μM | 17404006 | |||
| Huh7 | Antiviral assay | 5 days | Antiviral activity against HBV genotype D with reverse transcriptase L80I mutation in human Huh7 cells assessed as inhibition of viral replication after 5 days, EC50=0.03μM | 17438047 | ||
| Huh7 | Antiviral assay | 5 days | Antiviral activity against HBeAg-deficient HBV genotype D with precore G1896A mutation and reverse transcriptase L80I mutation in human Huh7 cells assessed as inhibition of viral replication after 5 days, EC50=0.03μM | 17438047 | ||
| Huh7 | Antiviral assay | 5 days | Antiviral activity against HBV genotype D in human Huh7 cells assessed as inhibition of viral replication after 5 days, EC50=0.41μM | 17438047 | ||
| Huh7 | Antiviral assay | 5 days | Antiviral activity against HBeAg-deficient HBV genotype D with precore G1896A mutation in human Huh7 cells assessed as inhibition of viral replication after 5 days, EC50=0.46μM | 17438047 | ||
| HepG2(2.2.15) | Antiviral assay | Antiviral activity against HBV in ayw wild type HepG2(2.2.15) cells assessed as inhibition of viral DNA replication, IC50=0.05μM | 17488817 | |||
| HepG2(2.2.15) | Function assay | Inhibition of HBV promoter in human HepG2(2.2.15) cells by luciferase reporter gene assay, IC50=0.08μM | 17488817 | |||
| HepW10 | Antiviral assay | Antiviral activity against HBV in adriamycin-resistant wild type HepW10 cells assessed as inhibition of viral DNA replication, IC50=0.1μM | 17488817 | |||
| HBV-Met | Antiviral assay | Antiviral activity against HBV in mouse ayw wild type HBV-Met cells assessed as inhibition of viral DNA replication, IC50=0.2μM | 17488817 | |||
| HepG2.2.15 | Function assay | 8 days | Inhibition of cytoplasmic HBV DNA synthesis in HepG2.2.15 cells after 8 days, IC50=0.38μM | 17499889 | ||
| PBMC | Function assay | 300 mg | 7 days | Cmax in HIV-infected human assessed as Lamivudine monophosphate level in PBMC at 300 mg QD for 7 days measured per 10'6 cells in presence of zidovudine, Cmax=0.0000008μM | 17664328 | |
| PBMC | Function assay | 300 mg | 7 days | Cmax in HIV-infected human assessed as Lamivudine diphosphate level in PBMC at 300 mg QD for 7 days measured per 10'6 cells in presence of zidovudine, Cmax=0.0000044μM | 17664328 | |
| PBMC | Function assay | 300 mg | 7 days | Cmax in HIV-infected human assessed as Lamivudine triphosphate level in PBMC at 300 mg QD for 7 days measured per 10'6 cells in presence of zidovudine, Cmax=0.0000048μM | 17664328 | |
| MAGIC-5A | Antiviral assay | 40 hrs | Antiviral activity against wild type HIV1 NL4-3 produced from full length pR9deltaApa infected in human MAGIC-5A cells assessed as inhibition of Lac+ foci expression after 40 hrs, EC50=0.88μM | 17967913 | ||
| MAGIC-5A | Antiviral assay | 40 hrs | Antiviral activity against wild type HIV2 ROD produced from full length pROD9 infected in human MAGIC-5A cells assessed as inhibition of Lac+ foci expression after 40 hrs, EC50=2μM | 17967913 | ||
| MAGIC-5A | Antiviral assay | 40 hrs | Antiviral activity against multinucleoside-resistant HIV1 NL4-3 harboring A62V, V75I, F77L, F116Y, Q151M mutation in viral reverse transcriptase infected in human MAGIC-5A cells assessed as inhibition of Lac+ foci expression after 40 hrs, EC50=3.7μM | 17967913 | ||
| PBMC | Antiviral assay | 1 hr | Antiviral activity against HIV1 subtype B-ASM 044 infected in 1 hr-pretreated PBMC cells assessed as inhibition of p24 antigen production measured on day 5 postinfection by ELISA, EC50=0.04μM | 18316521 | ||
| MT2 | Antiviral assay | 5 days | Antiviral activity against 0.005 MOI wild type HIV1 NL4-3 infected in human MT2 cells measured after 5 days by RT SPA, EC50=0.04μM | 18316521 | ||
| MT2 | Antiviral assay | Antiviral activity against 0.005 MOI HIV1 NL4-3 harboring wild type RT infected in human MT2 cells, EC50=0.04μM | 18316521 | |||
| MT2 | Antiviral assay | Antiviral activity against HIV1 subtype B-3B infected in 1 hr-pretreated human MT2 cells assessed as inhibition of multicycle replication measured on day 5 postinfection by RT SPA, EC50=0.047μM | 18316521 | |||
| MT2 | Antiviral assay | 5 days | Antiviral activity against 0.01 MOI wild type HIV1 NL4-3 infected in human MT2 cells measured after 5 days by RT SPA, EC50=0.063μM | 18316521 | ||
| PBMC | Antiviral assay | Antiviral activity against HIV1 subtype B-ASM 034 infected in 1 hr-pretreated PBMC cells assessed as inhibition of p24 antigen production measured on day 5 postinfection by ELISA, EC50=0.071μM | 18316521 | |||
| PBMC | Antiviral assay | Antiviral activity against HIV1 subtype B-92US076 infected in 1 hr-pretreated PBMC cells assessed as inhibition of p24 antigen production measured on day 5 postinfection by ELISA, EC50=0.081μM | 18316521 | |||
| MT2 | Antiviral assay | 5 days | Antiviral activity against 0.02 MOI wild type HIV1 NL4-3 infected in human MT2 cells measured after 5 days by RT SPA, EC50=0.086μM | 18316521 | ||
| MT2 | Antiviral assay | 1 hr | Antiviral activity against HIV1 subtype B-RF infected in 1 hr-pretreated human MT2 cells assessed as inhibition of multicycle replication measured on day 5 postinfection by RT SPA, EC50=0.105μM | 18316521 | ||
| MT2 | Antiviral assay | 1 hr | Antiviral activity against HIV1 subtype B-NL4-3 infected in 1 hr-pretreated human MT2 cells assessed as inhibition of multicycle replication measured on day 5 postinfection by RT SPA, EC50=0.171μM | 18316521 | ||
| MT2 | Antiviral assay | 1 hr | Antiviral activity against HIV1 subtype B-LAI infected in 1 hr-pretreated human MT2 cells assessed as inhibition of multicycle replication measured on day 5 postinfection by RT SPA, EC50=0.182μM | 18316521 | ||
| MT2 | Antiviral assay | 1 hr | Antiviral activity against HIV1 subtype B-SF-2 infected in 1 hr-pretreated human MT2 cells assessed as inhibition of multicycle replication measured on day 5 postinfection by RT SPA, EC50=0.183μM | 18316521 | ||
| MT2 | Antiviral assay | 1 hr | Antiviral activity against HIV1 subtype B-HXB2 infected in 1 hr-pretreated human MT2 cells assessed as inhibition of multicycle replication measured on day 5 postinfection by RT SPA, EC50=0.202μM | 18316521 | ||
| MT2 | Antiviral assay | 5 days | Antiviral activity against 0.005 MOI wild type HIV1 NL4-3 infected in human MT2 cells measured after 5 days by MTS assay, EC50=0.219μM | 18316521 | ||
| MT2 | Antiviral assay | 5 days | Antiviral activity against 0.05 MOI wild type HIV1 NL4-3 infected in human MT2 cells measured after 5 days by RT SPA, EC50=0.372μM | 18316521 | ||
| MT2 | Antiviral assay | 5 days | Antiviral activity against 0.005 MOI wild type HIV1 NL4-3 infected in 2 hr-pretreated human MT2 cells measured after 5 days by RT SPA, EC50=0.462μM | 18316521 | ||
| MT2 | Antiviral assay | 5 days | Antiviral activity against 0.01 MOI wild type HIV1 NL4-3 infected in human MT2 cells measured after 5 days by MTS assay, EC50=0.554μM | 18316521 | ||
| MT2 | Antiviral assay | 5 days | Antiviral activity against 0.01 MOI wild type HIV1 NL4-3 infected in 2 hr-pretreated human MT2 cells measured after 5 days by RT SPA, EC50=0.667μM | 18316521 | ||
| PBMC | Antiviral assay | 1 hr | Antiviral activity against HIV1 subtype B-93US143 infected in 1 hr-pretreated PBMC cells assessed as inhibition of p24 antigen production measured on day 5 postinfection by ELISA, EC50=0.85μM | 18316521 | ||
| MT2 | Antiviral assay | 5 days | Antiviral activity against 0.05 MOI wild type HIV1 NL4-3 infected in human MT2 cells measured after 5 days by MTS assay, EC50=0.927μM | 18316521 | ||
| MT2 | Antiviral assay | 5 days | Antiviral activity against 0.02 MOI wild type HIV1 NL4-3 infected in human MT2 cells measured after 5 days by MTS assay, EC50=0.969μM | 18316521 | ||
| MT2 | Antiviral assay | 5 days | Antiviral activity against pseudotype HIV1 NL-RLuc infected in human MT2 cells measured on day 5 postinfection by luciferase reporter gene assay, EC50=1.14μM | 18316521 | ||
| MT2 | Antiviral assay | 5 days | Antiviral activity against 0.005 MOI wild type HIV1 NL4-3 infected in 2 hr-pretreated human MT2 cells measured after 5 days by MTS assay, EC50=1.259μM | 18316521 | ||
| MT2 | Antiviral assay | 5 days | Antiviral activity against 0.01 MOI wild type HIV1 NL4-3 infected in 2 hr-pretreated human MT2 cells measured after 5 days by MTS assay, EC50=1.683μM | 18316521 | ||
| MT2 | Antiviral assay | 5 days | Antiviral activity against 0.02 MOI wild type HIV1 NL4-3 infected in 2 hr-pretreated human MT2 cells measured after 5 days by RT SPA, EC50=1.985μM | 18316521 | ||
| MT2 | Antiviral assay | 5 days | Antiviral activity against 0.02 MOI wild type HIV1 NL4-3 infected in 2 hr-pretreated human MT2 cells measured after 5 days by MTS assay, EC50=2.445μM | 18316521 | ||
| MT2 | Antiviral assay | 5 days | Antiviral activity against 0.05 MOI wild type HIV1 NL4-3 infected in 2 hr-pretreated human MT2 cells measured after 5 days by RT SPA, EC50=2.47μM | 18316521 | ||
| MT2 | Antiviral assay | 5 days | Antiviral activity against 0.05 MOI wild type HIV1 NL4-3 infected in 2 hr-pretreated human MT2 cells measured after 5 days by MTS assay, EC50=3.247μM | 18316521 | ||
| HepG2(2.2.15) | Antiviral assay | 24 hrs | Antiviral activity against HBV in human HepG2(2.2.15) cells assessed as extracellular HBV virion DNA level after 24 hrs, EC50=0.048μM | 19072694 | ||
| HepG2(2.2.15) | Antiviral assay | 24 hrs | Antiviral activity against HBV in human HepG2(2.2.15) cells assessed as replication intermediates after 24 hrs, EC50=0.15μM | 19072694 | ||
| Huh7 | Antiviral assay | 4 days | Antiviral activity against lamivudine-resistant wild type HBV in human Huh7 cells after 4 days by intracellular HBV DNA replication assay, EC50=0.2μM | 19072694 | ||
| Huh7 | Antiviral assay | 4 days | Antiviral activity against lamivudine-resistant HBV reverse transcriptase 236T mutant in human Huh7 cells after 4 days by intracellular HBV DNA replication assay, EC50=0.3μM | 19072694 | ||
| Huh7 | Antiviral assay | 4 days | Antiviral activity against adefovir-resistant wild type HBV in human Huh7 cells after 4 days by intracellular HBV DNA replication assay, EC50=1.3μM | 19072694 | ||
| Huh7 | Antiviral assay | 4 days | Antiviral activity against adefovir-resistant HBV reverse transcriptase M204V mutant in human Huh7 cells after 4 days by intracellular HBV DNA replication assay, EC50=1.5μM | 19072694 | ||
| Huh7 | Antiviral assay | 4 days | Antiviral activity against adefovir-resistant HBV reverse transcriptase L180M mutant in human Huh7 cells after 4 days by intracellular HBV DNA replication assay, EC50=1.6μM | 19072694 | ||
| Huh7 | Antiviral assay | 4 days | Antiviral activity against adefovir-resistant HBV reverse transcriptase LM/reverse transcriptase MV double mutant in human Huh7 cells after 4 days by intracellular HBV DNA replication assay, EC50=1.6μM | 19072694 | ||
| Huh7 | Antiviral assay | 4 days | Antiviral activity against adefovir-resistant HBV reverse transcriptase M204I mutant in human Huh7 cells after 4 days by intracellular HBV DNA replication assay, EC50=1.8μM | 19072694 | ||
| Huh7 | Antiviral assay | 4 days | Antiviral activity against adefovir-resistant HBV reverse transcriptase 236T mutant in human Huh7 cells after 4 days by intracellular HBV DNA replication assay, EC50=7.7μM | 19072694 | ||
| Huh7 | Antiviral assay | 4 days | Antiviral activity against lamivudine-resistant HBV reverse transcriptase L180M mutant in human Huh7 cells after 4 days by intracellular HBV DNA replication assay, EC50=10μM | 19072694 | ||
| HuH7 | Antiviral assay | 24 hrs | Antiviral activity against wild type HBV infected in human HuH7 cells assessed as decrease in viral transient transfection after 24 hrs, EC50=0.2μM | 19398648 | ||
| HuH7 | Antiviral assay | 24 hrs | Antiviral activity against HBV harboring RNA polymerase N236T mutant gene infected in human HuH7 cells assessed as decrease in viral transient transfection after 24 hrs, EC50=0.3μM | 19398648 | ||
| HuH7 | Antiviral assay | 24 hrs | Antiviral activity against HBV harboring RNA polymerase N236T mutant gene infected in human HuH7 cells assessed as decrease in viral transient transfection after 24 hrs, EC90=1.5μM | 19398648 | ||
| HuH7 | Antiviral assay | 24 hrs | Antiviral activity against wild type HBV infected in human HuH7 cells assessed as decrease in viral transient transfection after 24 hrs, EC90=3μM | 19398648 | ||
| HuH7 | Antiviral assay | 24 hrs | Antiviral activity against HBV harboring RNA polymerase L180M mutant gene infected in human HuH7 cells assessed as decrease in viral transient transfection after 24 hrs, EC50=16μM | 19398648 | ||
| HuH7 | Antiviral assay | 24 hrs | Antiviral activity against HBV harboring RNA polymerase L180M mutant gene infected in human HuH7 cells assessed as decrease in viral transient transfection after 24 hrs, EC90=41μM | 19398648 | ||
| MT2 | Antiviral assay | Antiviral activity against HIV1 infected in human MT2 cells assessed as inhibition of viral replication, IC50=0.6μM | 19596885 | |||
| HeLa P4/R5 | Antiviral assay | Antiviral activity against HIV1 infected in human HeLa P4/R5 cells assessed as inhibition of viral replication, IC50=0.78μM | 19596885 | |||
| TZM-bl | Antiviral assay | 72 hrs | Antiviral activity against HIV1 pNL4-3 harboring Reverse transcriptase P119S mutant gene infected in TZM-bl cells after 72 hrs by luciferase assay, EC50=0.47μM | 19704131 | ||
| TZM-bl | Antiviral assay | Antiviral activity against wild-type HIV1 pNL4-3 infected in TZM-bl cells by luciferase assay, EC50=0.65μM | 19704131 | |||
| TZM-bl | Antiviral assay | 72 hrs | Antiviral activity against HIV1 pNL4-3 harboring Reverse transcriptase T165A mutant gene infected in TZM-bl cells after 72 hrs by luciferase assay, EC50=1.08μM | 19704131 | ||
| TZM-bl | Antiviral assay | 72 hrs | Antiviral activity against HIV1 pNL4-3 harboring Reverse transcriptase P119S/T165A mutant gene infected in TZM-bl cells after 72 hrs by luciferase assay, EC50=2.1μM | 19704131 | ||
| HepG2.2.15 | Antiviral assay | Antiviral activity against HBV infected in human HepG2.2.15 cells assessed as inhibition of viral DNA replication, IC50=0.22μM | 19897363 | |||
| 1.3ES2 | Antiviral assay | 6 days | Antiviral activity against Hepatitis B virus ayw in human 1.3ES2 cells stably transfected with 1.3 fold wild type HBV ayw strain genome cells assessed as reduction of viral DNA level after 6 days by RT-PCR | 20061158 | ||
| HepG2.2.15 | Antiviral assay | 9 days | Antiviral activity against wild type HBV infected in HepG2.2.15 cells assessed as nucleic acid and protein levels dosed daily for 9 days and measured 24 hrs after last treatment by Southern blot hybridization, EC50=0.2μM | 20117930 | ||
| HepG2.2.15 | Antiviral assay | 9 days | Antiviral activity against ADV-resistant HBV N236T mutation infected in HepG2.2.15 cells assessed as nucleic acid and protein levels dosed daily for 9 days and measured 24 hrs after last treatment by Southern blot hybridization, EC50=0.2μM | 20117930 | ||
| HepG2.2.15 | Antiviral assay | 9 days | Antiviral activity against wild type HBV infected in HepG2.2.15 cells assessed as nucleic acid and protein levels dosed daily for 9 days and measured 24 hrs after last treatment by Southern blot hybridization, EC90=0.7μM | 20117930 | ||
| HepG2.2.15 | Antiviral assay | 9 days | Antiviral activity against ADV-resistant HBV N236T mutation infected in HepG2.2.15 cells assessed as nucleic acid and protein levels dosed daily for 9 days and measured 24 hrs after last treatment by Southern blot hybridization, EC90=0.8μM | 20117930 | ||
| HepG2.2.15 | Antiviral assay | 9 days | Antiviral activity against 3TC-resistant HBV with polymerase L180M mutation infected in HepG2.2.15 cells assessed as nucleic acid and protein levels dosed daily for 9 days and measured 24 hrs after last treatment by Southern blot hybridization, EC50=5.3μM | 20117930 | ||
| HepG2.2.15 | Antiviral assay | 9 days | Antiviral activity against 3TC-resistant HBV with polymerase L180M mutation infected in HepG2.2.15 cells assessed as nucleic acid and protein levels dosed daily for 9 days and measured 24 hrs after last treatment by Southern blot hybridization, EC90=20μM | 20117930 | ||
| HepG2(2.2.15) | Antiviral assay | 13 uM | 72 hrs | Antiviral activity against Hepatitis B virus infected in human HepG2(2.2.15) cells assessed as decrease in intracellular viral DNA level at 13 uM after 72 hrs by RT-PCR analysis | 20176893 | |
| HEK293 | Antiviral assay | 72 hrs | Antiviral activity against HIV1 subtype D isolate 8 infected in HEK293 cells assessed as inhibition of virus replication after 72 hrs, EC50=3.25μM | 20308377 | ||
| HEK293 | Antiviral assay | 72 hrs | Antiviral activity against HIV1 subtype A isolate 1 infected in HEK293 cells assessed as inhibition of virus replication after 72 hrs, EC50=3.54μM | 20308377 | ||
| HEK293 | Antiviral assay | 72 hrs | Antiviral activity against HIV1 subtype C isolate 6 infected in HEK293 cells assessed as inhibition of virus replication after 72 hrs, EC50=3.9μM | 20308377 | ||
| HEK293 | Antiviral assay | 72 hrs | Antiviral activity against HIV1 subtype C isolate 7 infected in HEK293 cells assessed as inhibition of virus replication after 72 hrs, EC50=4.06μM | 20308377 | ||
| HEK293 | Antiviral assay | 72 hrs | Antiviral activity against HIV1 subtype BF isolate 5 infected in HEK293 cells assessed as inhibition of virus replication after 72 hrs, EC50=4.24μM | 20308377 | ||
| HEK293 | Antiviral assay | 72 hrs | Antiviral activity against HIV1 subtype A isolate 2 infected in HEK293 cells assessed as inhibition of virus replication after 72 hrs, EC50=4.48μM | 20308377 | ||
| HEK293 | Antiviral assay | 72 hrs | Antiviral activity against HIV1 NL4-3 infected in HEK293 cells assessed as inhibition of virus replication after 72 hrs, EC50=4.68μM | 20308377 | ||
| HEK293 | Antiviral assay | 72 hrs | Antiviral activity against HIV1 subtype B isolate 3 infected in HEK293 cells assessed as inhibition of virus replication after 72 hrs, EC50=4.78μM | 20308377 | ||
| HEK293 | Antiviral assay | 72 hrs | Antiviral activity against HIV1 subtype B isolate 4 infected in HEK293 cells assessed as inhibition of virus replication after 72 hrs, EC50=5.43μM | 20308377 | ||
| HEK293 | Antiviral assay | 72 hrs | Antiviral activity against HIV1 expressing reverse transcriptase K103N/Y181C/G190A mutant infected in HEK293 cells assessed as inhibition of virus replication after 72 hrs, EC50=7.16μM | 20308377 | ||
| HepG2(2.2.15) | Antiviral assay | 8 days | Antiviral activity against Hepatitis B virus infected in human HepG2(2.2.15) cells assessed as inhibition of viral cytoplasmic DNA synthesis after 8 days by RT-PCR analysis, IC50=0.16μM | 20639110 | ||
| HepG2(2.2.15) | Cytotoxicity assay | 8 days | Cytotoxicity against human HepG2(2.2.15) cells after 8 days by MTT assay, CC50=5μM | 20639110 | ||
| MT2 | Antiviral assay | 2 to 3 days | Antiviral activity against Human immunodeficiency virus 1 3B infected in human MT2 cells assessed as inhibition of virus induced cytopathic effect measured after 2 to 3 days by PCR, EC50=0.8μM | 21115794 | ||
| MT2 | Antiviral assay | Antiviral activity against Human immunodeficiency virus 1 3B harboring reverse transcriptase M184V, M184I mutant infected in human MT2 cells assessed as inhibition of virus induced cytopathic effect selected on day 14 after 4 passages by PCR analysis, EC50=0.8μM | 21115794 | |||
| MT2 | Antiviral assay | Antiviral activity against Human immunodeficiency virus 1 3B harboring reverse transcriptase M184V mutant infected in human MT2 cells assessed as inhibition of virus induced cytopathic effect selected on day 14 after 4 passages by PCR analysis, EC50=0.8μM | 21115794 | |||
| MT2 | Antiviral assay | Antiviral activity against Human immunodeficiency virus 1 3B harboring reverse transcriptase M184V, M184I, K82N mutant infected in human MT2 cells assessed as inhibition of virus induced cytopathic effect selected on day 14 after 4 passages by PCR analysi, EC50=0.8μM | 21115794 | |||
| HepG2(2.2.15) | Antiviral assay | Antiviral activity against Hepatitis B virus infected in human HepG2(2.2.15) cells assessed as reduction in viral DNA by Southern blotting, EC50=0.06μM | 21333535 | |||
| Huh7 | Antiviral assay | 1 day | Antiviral activity against Hepatitis B virus infected in human Huh7 cells assessed as reduction in viral DNA after 1 day by Southern blotting, EC50=0.1μM | 21333535 | ||
| HuH7 | Cytotoxicity assay | 4 days | Cytotoxicity against human HuH7 cells after 4 days by neutral red dye uptake assay, CC50=0.1μM | 21333535 | ||
| HepG2(2.2.15) | Antiviral assay | 4 days | Antiviral activity against Hepatitis B virus infected in human HepG2(2.2.15) cells assessed as inhibition of viral DNA replication after 4 days by RT-PCR analysis, IC50=0.33μM | 21524588 | ||
| HepG2(2.2.15) | Antiviral assay | 9 days | Antiviral activity against wild type Hepatitis B virus infected in human HepG2(2.2.15) cells assessed as inhibition of virus replication treated daily for 9 days by quantitative blot hybridization method, EC50=0.2μM | 21930377 | ||
| HepG2(2.2.15) | Antiviral assay | 9 days | Antiviral activity against adefovir-resistant Hepatitis B virus harboring reverse transcriptase N236T mutant infected in human HepG2(2.2.15) cells assessed as inhibition of virus replication treated daily for 9 days by quantitative blot hybridization meth, EC50=0.2μM | 21930377 | ||
| HepG2(2.2.15) | Antiviral assay | 9 days | Antiviral activity against wild type Hepatitis B virus infected in human HepG2(2.2.15) cells assessed as reduction of infectious virus titer treated daily for 9 days by quantitative Southern blot hybridization assay, EC90=0.6μM | 21930377 | ||
| HepG2(2.2.15) | Antiviral assay | 9 days | Antiviral activity against adefovir-resistant Hepatitis B virus harboring reverse transcriptase N236T mutant infected in human HepG2(2.2.15) cells assessed as reduction of infectious virus titer treated daily for 9 days by quantitative Southern blot hybri, EC90=0.9μM | 21930377 | ||
| HepG2(2.2.15) | Antiviral assay | 9 days | Antiviral activity against lamivudine-resistant Hepatitis B virus harboring reverse transcriptase L180M mutant infected in human HepG2(2.2.15) cells assessed as inhibition of virus replication treated daily for 9 days by quantitative blot hybridization me, EC50=1.5μM | 21930377 | ||
| HepG2(2.2.15) | Antiviral assay | 9 days | Antiviral activity against lamivudine-resistant Hepatitis B virus harboring reverse transcriptase L180M mutant infected in human HepG2(2.2.15) cells assessed as reduction of infectious virus titer treated daily for 9 days by quantitative Southern blot hyb, EC90=22μM | 21930377 | ||
| HeLa P4/R5 | Antiviral assay | 2 hrs | Antiviral activity against HIV1 BaL infected in HeLa P4/R5 cells assessed as reduction of viral infection incubated for 2 hrs followed by incubated in drug-free medium for 46 hrs by single round infection beta-galactosidase reporter gene assay, EC50=11.3μM | 22352809 | ||
| HeLa P4/R5 | Antiviral assay | 2 hrs | Antiviral activity against HIV1 3B infected in HeLa P4/R5 cells assessed as reduction of viral infection incubated for 2 hrs followed by incubated in drug-free medium for 46 hrs by single round infection beta-galactosidase reporter gene assay, EC50=32.7μM | 22352809 | ||
| HeLa P4/R5 | Antiviral assay | 2 hrs | Antiviral activity against HIV1 BaL assessed as inhibition of infection in HeLa P4/R5 cells expressing CD4 receptor and beta-gal incubated for 2 hrs followed by compound-wash out measured after 46 hrs by single round infection MAGI assay, EC50=11.4μM | 22533850 | ||
| HeLa P4/R5 | Antiviral assay | 2 hrs | Antiviral activity against HIV1 3B assessed as inhibition of infection in HeLa P4/R5 cells expressing CD4 receptor and beta-gal incubated for 2 hrs followed by compound-wash out measured after 46 hrs by single round infection MAGI assay, EC50=32.7μM | 22533850 | ||
| CEM-SS | Antiviral assay | 6 days | Antiviral activity against HIV-1 subtype 3B infected in CEM-SS cells assessed as inhibition of viral replication after 6 days by XTT assay, EC50=0.2μM | 22858097 | ||
| HuH7 | Antiviral assay | Antiviral activity against wild type Hepatitis B virus infected in human HuH7 cells, EC50=0.056μM | 23237841 | |||
| HuH7 | Antiviral assay | Antiviral activity against wild type Hepatitis B virus infected in human HuH7 cells, EC90=0.142μM | 23237841 | |||
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV1 3B infected in human MT4 cells assessed as protection against viral-induced cytotoxicity after 5 days by MTT assay, EC50=1.8μM | 24119448 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV2 ROD infected in human MT4 cells assessed as protection against viral-induced cytotoxicity after 5 days by MTT assay, EC50=5.9μM | 24119448 | ||
| CEM | Antiviral assay | 4 days | Antiviral activity against HIV1 3B infected in human CEM cells assessed as virus-induced giant cell formation after 4 days by microscopic analysis, EC50=0.099μM | 24177359 | ||
| CEM | Antiviral assay | 4 days | Antiviral activity against HIV2 ROD infected in human CEM cells assessed as virus-induced giant cell formation after 4 days by microscopic analysis, EC50=0.18μM | 24177359 | ||
| HOG.R5 | Antiviral assay | Antiviral activity against HIV-1 3B/H9 infected in human HOG.R5 cells transfected with HIV1 LTR-GFP assessed as inhibition of viral replication by fluorescence analysis, IC50=1.2μM | 24404757 | |||
| MT4 | Antiviral assay | Antiviral activity against HIV1 3B infected in human MT4 cells assessed as inhibition of virus-induced cytopathicity measured 5 days post viral infection by MTT assay, EC50=2.442μM | 24794751 | |||
| MT4 | Antiviral assay | Antiviral activity against HIV2 ROD infected in human MT4 cells assessed as inhibition of virus-induced cytopathicity measured 5 days post viral infection by MTT assay, EC50=7.2μM | 24794751 | |||
| MT4 | Antiviral assay | 5 days | Antiviral activity against Human immunodeficiency virus 1 3b infected in human MT4 cells assessed as inhibition of virus replication after 5 days by MTT assay, IC50=3.9μM | 24926807 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against Human immunodeficiency virus type 2 ROD infected in human MT4 cells assessed as inhibition of virus replication after 5 days by MTT assay, IC50=15.5μM | 24926807 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against wild type HIV1 3B infected in human MT4 cells assessed as inhibition of virus-induced cytopathogenicity after 5 days by MTT assay, IC50=3.89μM | 24952305 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV2 ROD infected in human MT4 cells assessed as inhibition of virus-induced cytopathogenicity after 5 days by MTT assay, IC50=15.53μM | 24952305 | ||
| HepG2.2.15 | Antiviral assay | Antiviral activity against hepatitis B virus infected in human HepG2.2.15 cells assessed as inhibition of viral replication, IC50=14.17μM | 25127104 | |||
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV1 3B infected in human MT4 cells assessed as inhibition of virus-induced syncytium formation after 5 days by MTT assay, EC50=2.22μM | 25240095 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV2 ROD infected in human MT4 cells assessed as inhibition of virus-induced syncytium formation after 5 days by MTT assay, EC50=8.81μM | 25240095 | ||
| HepG2(2.2.15) | Function assay | 0.75 to 3 uM | 72 hrs | Decrease in HBV viral DNA replication in human HepG2(2.2.15) cells at 0.75 to 3 uM after 72 hrs by real-time PCR analysis, IC50=7.63μM | 25461891 | |
| MT4 | Antiviral assay | 5 days | Antiviral activity against wild type HIV-1 3B infected in human MT4 cells assessed as reduction of virus-induced cytopathic effect after 5 days by MTT assay, EC50=2.24μM | 25537532 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against wild type HIV-2 ROD infected in human MT4 cells assessed as reduction of virus-induced cytopathic effect after 5 days by MTT assay, EC50=8.79μM | 25537532 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV1 3B infected in human MT4 cells assessed as reduction in virus-induced cytopathic effect after 5 days by MTT assay, EC50=2.239μM | 25626145 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV1 3B infected infected in human MT4 cells assessed as protection against virus-induced cytotoxicity after 5 days by MTT assay, EC50=0.89μM | 25682562 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV2 ROD infected in human MT4 cells assessed as protection against virus-induced cytotoxicity after 5 days by MTT assay, EC50=3.56μM | 25682562 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV1 3B infected in human MT4 cells assessed as reduction in virus-induced cytopathic effect after 5 days by MTT assay, EC50=2.2μM | 25707013 | ||
| HepG2.2.15 | Antiviral assay | Antiviral activity against Hepatitis B virus infected in human HepG2.2.15 cells assessed as inhibition of HBV DNA replication by real-time fluorescence quantitative PCR analysis, IC50=6.86μM | 25847765 | |||
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV1-3B infected in human MT4 cells assessed as inhibition of virus induced cell death measured after 5 days of infection by MTT assay, EC50=3.9μM | 25946116 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV2-ROD infected in human MT4 cells assessed as inhibition of virus induced cell death measured after 5 days of infection by MTT assay, EC50=15.5μM | 25946116 | ||
| HepG22.2.15 | Antiviral assay | 3 days | Antiviral activity against HBV infected in human HepG22.2.15 cells after 3 days by neutral red dye-based plaque reduction assay, IC50=0.039μM | 26260336 | ||
| U87 | Antiviral assay | 10 uM | 4 days | Antiviral activity against HIV-1 NL4-3 infected in human U87 cells expressing CD4.CXCR4 assessed as inhibition of p24 production at 10 uM measured after 4 days by p24 ELISA | 27091070 | |
| U87 | Antiviral assay | 100 uM | 4 days | Antiviral activity against HIV-1 NL4-3 infected in human U87 cells expressing CD4.CXCR4 assessed as inhibition of p24 production at 100 uM measured after 4 days by p24 ELISA | 27091070 | |
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV1 3B expressing wild type reverse transcriptase infected in human MT4 cells assessed as protection against virus-induced cytopathic effect after 5 days by MTT assay, EC50=3.1μM | 27267005 | ||
| HepG2.2.15.7 | Antiviral assay | 9 days | Antiviral activity against HBV infected in human HepG2.2.15.7 cells assessed as inhibition of viral DNA level after 9 days by real time PCR method, EC50=0.03μM | 27426303 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against wild type HIV1 3B harboring reverse transcriptase infected in human MT4 cells assessed as protection against virus induced cytotoxicity after 5 days by MTT assay, EC50=2.24μM | 27541578 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV2 ROD harboring reverse transcriptase infected in human MT4 cells assessed as protection against virus induced cytotoxicity after 5 days by MTT assay, EC50=8.79μM | 27541578 | ||
| HepG2(2.2.15) | Antiviral assay | 6 days | Antiviral activity against HBV ayw1 infected in human HepG2(2.2.15) cells assessed as reduction in viral replication by measuring intracellular viral DNA copy number after 6 days by real-time qPCR method, EC50<0.01μM | 27748590 | ||
| HepG2(2.2.15) | Antiviral assay | 6 days | Antiviral activity against HBV ayw1 infected in human HepG2(2.2.15) cells assessed as reduction in viral replication by measuring extracellular viral DNA copy number after 6 days by real-time qPCR method, EC50=0.01μM | 27748590 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV1 3B infected in human MT4 cells assessed as protection against virus-induced cytopathic effect after 5 days by MTT assay, EC50=2.22μM | 27748590 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV2 ROD infected in human MT4 cells assessed as protection against virus-induced cytopathic effect after 5 days by MTT assay, EC50=8.81μM | 27748590 | ||
| Huh7 | Antiviral assay | Antiviral activity against lamivudine-resistant wild type hepatitis B virus infected in human Huh7 cells assessed as reduction in secreted DNA levels measured on day 7 by real-time PCR analysis, EC50=0.055μM | 28082068 | |||
| HepG2.215 | Antiviral assay | Antiviral activity against hepatitis B virus infected in human HepG2.215 cells assessed as reduction in viral DNA replication measured on day 7 by real time PCR analysis, EC50=0.1μM | 28082068 | |||
| Huh7 | Antiviral assay | Antiviral activity against lamivudine-resistant wild type hepatitis B virus infected in human Huh7 cells assessed as reduction in intracellular DNA levels measured on day 7 by real-time PCR analysis, EC50=0.237μM | 28082068 | |||
| HepG2.215 | Antiviral assay | Antiviral activity against hepatitis B virus infected in human HepG2.215 cells assessed as reduction in viral DNA replication measured on day 7 by real time PCR analysis, EC90=2μM | 28082068 | |||
| HepAD38 | Antiviral assay | 5 days | Antiviral activity against HBV infected in human HepAD38 cells assessed as inhibition of DNA replication after 5 days by RT-PCR method, EC50=0.04μM | 28094179 | ||
| P4R5 MAGI | Function assay | 2 hrs | Inhibition of HIV1 BaL dinucleoside reverse transcriptase infected in human P4R5 MAGI cells assessed as reduction in viral infection after 2 hrs by Galacto-Star beta-galactosidase reporter gene assay, EC50=11.3μM | 28351588 | ||
| P4R5 MAGI | Function assay | 2 hrs | Inhibition of HIV1 3B dinucleoside reverse transcriptase infected in human P4R5 MAGI cells assessed as reduction in viral infection after 2 hrs by Galacto-Star beta-galactosidase reporter gene assay, EC50=32.7μM | 28351588 | ||
| HepG2.2.15 | Antiviral assay | 8 days | Antiviral activity against Hepatitis B virus infected in human HepG2.2.15 cells assessed as inhibition of viral DNA replication after 8 days by real time fluorescent PCR method, IC50=0.5μM | 28494252 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against wild type HIV1 3B infected in human MT4 cells assessed as inhibition of virus-induced cytopathic effect after 5 days by MTT assay, EC50=2.54μM | 28659246 | ||
| HepG2(2.2.15) | Antiviral assay | 6 days | Antiviral activity against HBV ayw1 infected in human HepG2(2.2.15) cells assessed as reduction in viral replication by measuring intracellular viral DNA copy number after 6 days byr by real-time qPCR assay, EC50=0.00887μM | 28682067 | ||
| HepG2(2.2.15) | Antiviral assay | 6 days | Antiviral activity against HBV ayw1 infected in human HepG2(2.2.15) cells assessed as reduction in viral replication by measuring extracellular viral DNA copy number after 6 days by real-time qPCR method, EC50=0.02μM | 28682067 | ||
| HepAD38 | Antiviral assay | Antiviral activity against HBV infected in human HepAD38 cells assessed as reduction in intracellular viral DNA level by RT-PCR method, EC50<0.01μM | 28688280 | |||
| HepAD38 | Antiviral assay | Antiviral activity against HBV infected in human HepAD38 cells assessed as reduction in viral replication by RT-PCR method, EC50=0.04μM | 28688280 | |||
| HepAD38 | Antiviral assay | Antiviral activity against HBV infected in human HepAD38 cells assessed as reduction in extracellular viral DNA level by RT-PCR method, EC50=0.04μM | 28688280 | |||
| HepAD38 | Antiviral assay | Antiviral activity against HBV infected in human HepAD38 cells assessed as reduction in viral replication by RT-PCR method, EC90=0.3μM | 28688280 | |||
| HepAD38 | Antiviral assay | Antiviral activity against HBV infected in human HepAD38 cells assessed as reduction in extracellular viral DNA level by RT-PCR method, EC90=0.3μM | 28688280 | |||
| HepAD38 | Antiviral assay | Antiviral activity against HBV infected in human HepAD38 cells assessed as reduction in intracellular viral DNA level by RT-PCR method, EC90=0.7μM | 28688280 | |||
| SupT1 | Antiviral assay | 48 hrs | Antiviral activity against VSV-G pseudotyped HIV1 infected in human SupT1 cells after 48 hrs by luciferase reporter gene assay, IC50=0.1μM | 29016131 | ||
| HepG2(2.2.15) | Antiviral assay | 6 days | Antiviral activity against HBV infected in human HepG2(2.2.15) cells assessed as inhibition of viral DNA replication after 6 days by RT-PCR method, IC50<0.1μM | 29288943 | ||
| A549 | Antiviral assay | Antiviral activity against HIV1 virions derived from HIV/VSG-G or HIV/HA virions infected 293T cells using human A549 cells as target cells assessed as inhibition of viral replication using compound pre-mixed virions followed by incubation with target cel, IC50=0.51μM | 29699924 | |||
| HepG2.2.15 | Antiviral assay | 6 days | Antiviral activity against Hepatitis B virus infected in human HepG2.2.15 cells assessed as reduction in extracellular viral DNA levels after 6 days by qPCR/TaqMan assay, IC50=0.025μM | 30286292 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV1 RES056 containing reverse transcriptase K103N + Y181C mutant infected in human MT4 cells assessed as inhibition of virus-induced cytopathic effect after 5 days by MTT assay, EC50=7.77μM | 30606670 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against wild type HIV1 3B infected in human MT4 cells assessed as inhibition of virus-induced cytopathic effect after 5 days by MTT assay, EC50=12.8μM | 30606670 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV1 3B infected in human MT4 cells assessed as inhibition of virus-induced cytotoxicity after 5 days by MTT assay, EC50=6.41μM | 30721060 | ||
| HepG2.2.15 | Function assay | 4 uM | 6 days | Inhibition of capsid protein in Hepatitis B virus infected in human HepG2.2.15 cells assessed as reduction in viral DNA level at 4 uM supplemented with fresh medium containing compound every 2 days and measured after 6 days by RT-PCR analysis, IC50<0.1μM | 31091479 | |
| HepG2.2.15 | Antiviral assay | Antiviral activity against HBV infected in human HepG2.2.15 cells assessed as reduction in cytoplasmic DNA synthesis by reed and munch method, IC50=2.22μM | 31223460 | |||
| C8166 | Antiviral assay | 72 hrs | Antiviral activity against HIV1 3B infected in human C8166 cells assessed as inhibition of syncytial cell formation incubated for 72 hrs, EC50=0.3μM | 31310115 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against drug resistant HIV-1 harboring reverse transcriptase F227L/V106A double mutant infected in human MT4 cells assessed as reduction in virus-induced cytopathic effect incubated for 5 days by MTT assay, EC50=1.92μM | 31434039 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against drug resistant HIV-1 harboring reverse transcriptase L100I mutant infected in human MT4 cells assessed as reduction in virus-induced cytopathic effect incubated for 5 days by MTT assay, EC50=3.14μM | 31434039 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against drug resistant HIV-1 harboring reverse transcriptase E138K mutant infected in human MT4 cells assessed as reduction in virus-induced cytopathic effect incubated for 5 days by MTT assay, EC50=3.58μM | 31434039 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against drug resistant HIV-1 harboring reverse transcriptase K103N mutant infected in human MT4 cells assessed as reduction in virus-induced cytopathic effect incubated for 5 days by MTT assay, EC50=3.7μM | 31434039 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV-1 3B infected in human MT4 cells assessed as reduction in virus-induced cytopathic effect incubated for 5 days by MTT assay, EC50=3.88μM | 31434039 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against drug resistant HIV-1 harboring reverse transcriptase Y188L mutant infected in human MT4 cells assessed as reduction in virus-induced cytopathic effect incubated for 5 days by MTT assay, EC50=4.45μM | 31434039 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against drug resistant HIV-1 harboring reverse transcriptase Y181C mutant infected in human MT4 cells assessed as reduction in virus-induced cytopathic effect incubated for 5 days by MTT assay, EC50=4.54μM | 31434039 | ||
| MT4 | Antiviral assay | 5 days | Antiviral activity against drug resistant HIV-1 RES056 harboring reverse transcriptase K103N/Y181C double mutant infected in human MT4 cells assessed as reduction in virus-induced cytopathic effect incubated for 5 days by MTT assay, EC50=5.54μM | 31434039 | ||
| PBMC | Antiviral assay | Antiviral activity in PBM cells expressed as IC50, IC50=0.002μM | ChEMBL | |||
| HepG2.2.15 | Antiviral assay | Antiviral activity against hepatitis B virus in human hepatoma cell line (2.2.15 cells), EC50=0.01μM | ChEMBL | |||
| HepG2.2.15 | Function assay | Effective concentration necessary to decrease extracellular Hepatitis B virus DNA levels by 50% in human hepatoblastoma cell line (2.2.15 cells) in vitro, EC50=0.038μM | ChEMBL | |||
| PBMC | Function assay | Compound was tested for its anti-HIV activity against HIV-1 LAI in human peripheral blood mononuclear (PBM) cells, EC50=0.06μM | ChEMBL | |||
| HepG2.2.15 | Function assay | Effective concentration necessary to decrease extracellular Hepatitis B virus DNA levels by 90% in human hepatoblastoma cell line (2.2.15 cells) in vitro, EC90=0.16μM | ChEMBL | |||
| HepG2.2.15 | Function assay | Tested for the effective concentration required to inhibit HBV in HepG2.2.15 human liver cells, EC50=0.2μM | ChEMBL | |||
| PBMC | Antiviral assay | Antiviral activity in PBM cells, IC50=0.21μM | ChEMBL | |||
| PBMC | Antiviral assay | Antiviral activity in PBM cells expressed as IC50, IC50=0.21μM | ChEMBL | |||
| MT-4 | Antiviral assay | Antiviral activity in MT-4 cells, IC50=0.37μM | ChEMBL | |||
| MT-4 | Antiviral assay | Antiviral activity in MT-4 cells expressed as IC50, IC50=0.37μM | ChEMBL | |||
| MT-4 | Antiviral assay | Antiviral activity in MT-4 cells expressed as IC50, IC50=0.61μM | ChEMBL | |||
| MT-4 | Function assay | Compound was tested for its anti-HIV activity in acutely infected MT-4 cells, EC50=7μM | ChEMBL | |||
| 클릭하여 더 많은 세포주 실험 데이터 보기 | ||||||
화학 정보, 보관 및 안정성
| 분자량 | 229.26 | 화학식 | C8H11N3O3S |
보관 (수령일로부터) | |
|---|---|---|---|---|---|
| CAS 번호 | 134678-17-4 | SDF 다운로드 | 원액 보관 |
|
|
| 동의어 | GR109714X, BCH-189 | Smiles | C1C(OC(S1)CO)N2C=CC(=NC2=O)N | ||
용해도
|
In vitro |
DMSO
: 45 mg/mL
(196.28 mM)
Water : 45 mg/mL Ethanol : Insoluble |
몰농도 계산기
|
In vivo |
|||||
생체 내 제형 계산기 (투명한 용액)
1단계: 아래 정보 입력 (권장: 실험 중 손실을 고려하여 추가 동물 포함)
2단계: 생체 내 제형 입력 (이것은 계산기일 뿐 제형이 아닙니다. 용해도 섹션에 생체 내 제형이 없는 경우 먼저 당사에 문의하십시오.)
계산 결과:
작업 농도: mg/ml;
DMSO 원액 준비 방법: mg 약물 사전 용해 μL DMSO ( 원액 농도 mg/mL, 농도가 해당 약물 배치의 DMSO 용해도를 초과하는 경우 먼저 당사에 문의하십시오. )
생체 내 제형 준비 방법: 취하다 μL DMSO 원액, 다음 추가μL PEG300, 혼합하고 투명하게 한 다음 추가μL Tween 80, 혼합하고 투명하게 한 다음 추가 μL ddH2O, 혼합하고 투명하게 합니다.
생체 내 제형 준비 방법: 취하다 μL DMSO 원액, 다음 추가 μL 옥수수 기름, 혼합하고 투명하게 합니다.
참고: 1. 다음 용매를 추가하기 전에 액체가 투명한지 확인하십시오.
2. 용매를 순서대로 추가해야 합니다. 다음 용매를 추가하기 전에 이전 추가에서 얻은 용액이 투명한 용액인지 확인해야 합니다. 와동, 초음파 또는 뜨거운 물 중탕과 같은 물리적 방법을 사용하여 용해를 도울 수 있습니다.
작용 메커니즘
| Targets/IC50/Ki |
Reverse transcriptase
|
|---|---|
| 시험관 내(In vitro) |
Lamivudine의 항-HBV 활성은 항-HIV 활성과 마찬가지로 LMV-TP가 HBV P 유전자 산물의 DNA 및 RNA 의존성 중합효소 활성의 기질이자 억제제 역할을 할 수 있는 능력에 따라 달라지는 것으로 나타났습니다. 이 화합물은 데옥시시티딘 키나제의 놀랍도록 넓은 기질 특이성과 비정상적인 L-형태를 가진 dNTP에 대한 HBV 중합효소의 특이한 기질 선호도 덕분에 활성을 나타내며, 반면에 PCV의 항-HBV 활성은 핵심 세포 효소에 의한 최적의 인산화(항바이러스 활성에는 충분하지만 세포독성에는 미치지 못함), PCV-TP의 긴 세포내 반감기, 그리고 HBV RT 프라이밍 반응 및 RT 및 DNA 중합효소 활성을 억제하는 PCV-TP의 능력 등 여러 요인에 따라 달라지는 것으로 보입니다. 이 화합물과 Penciclovir는 단독으로 사용했을 때 오리B형 간염 바이러스(DHBV) 복제를 비슷한 정도로 억제하며, 병용 시 두 뉴클레오사이드 유사체는 임상적으로 관련 있는 넓은 농도 범위에서 시너지 효과를 나타냅니다. Penciclovir와 결합하면 DHBV의 일반적으로 치료하기 어려운 바이러스성 공유 결합 폐쇄 원형(CCC) DNA 형태를 줄이는 데 단독 약물보다 더 효과적입니다. 이는 PBMC에서 HIV-I에 의한 p24 항원 생성을 억제하며, ED50은 0.07 μM에서 0.2 μM 범위입니다. |
참조 |
|
임상시험 정보
(데이터 출처 https://clinicaltrials.gov, 업데이트 날짜 2024-05-22)
| NCT 번호 | 모집 | 조건 | 스폰서/협력자 | 시작일 | 단계 |
|---|---|---|---|---|---|
| NCT06056596 | Recruiting | Retinal Detachment|Rhegmatogenous Retinal Detachment |
University of Wisconsin Madison |
January 30 2024 | Early Phase 1 |
| NCT04133012 | Completed | HIV-1 Infection |
ANRS Emerging Infectious Diseases|ViiV Healthcare |
February 10 2020 | Not Applicable |
