연구용
Melphalan DNA alkylator 화학물
제품 번호S8266
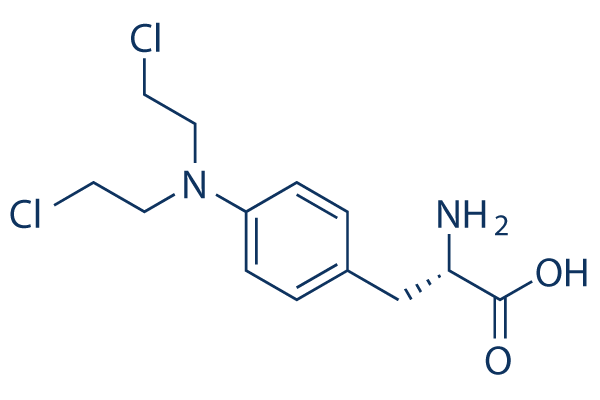
화학 구조
분자량: 305.20
품질 관리
세포 배양, 처리 및 작업 농도
| 세포주 | 분석 유형 | 농도 | 배양 시간 | 제형 | 활성 설명 | PMID |
|---|---|---|---|---|---|---|
| C6 (Rat) Glioma cell lines | Cytotoxicity assay | The compound was tested for cytotoxicity against C6 (Rat) Glioma cell lines, IC50=11 μM | ||||
| CEM T-lymphocytes | Cytotoxicity assay | Cytotoxicity against CEM T-lymphocytes, IC50=2.47 μM | ||||
| D283 MR (human) Glioma cell lines | Cytotoxicity assay | The compound was tested for cytotoxicity against D283 MR (human) Glioma cell lines, IC50=16.3 μM | ||||
| D283 (human) Glioma cell lines | Cytotoxicity assay | The compound was tested for cytotoxicity against D283 (human) Glioma cell lines, IC50=6.8 μM | ||||
| D341 (human) Glioma cell lines | Cytotoxicity assay | The compound was tested for cytotoxicity against D341 (human) Glioma cell lines, IC50=12.4 μM | ||||
| K562 | Cytotoxicity assay | 1 h | In vitro cytotoxic activity against human leukemic cell line K562 after incubation for 1 hour, IC50=30 μM | |||
| Jurkat T cells | Cytotoxicity assay | Inhibitory concentration against Human Jurkat T cells, IC50=2.2 μM | ||||
| L1210 cell | Cytotoxicity assay | 72 h | Tested in vitro for the cytotoxicity as number of viable cells against L1210 cell line after 72 hr treatment at conc. of 10E-6, ID50=1.7 μM | |||
| LoVo cell | Growth inhibition assay | 144 hr | Tested in vitro for inhibition after 144 hr exposure against human colon carcinoma LoVo cell line, IC50=4.09 μM | |||
| MCF-7 cells | Cytotoxicity assay | In vitro cytotoxicity activity against MCF-7, IC50=0.3 μM | ||||
| Molt 4/C8 cells | Cytotoxicity assay | Cytotoxicity against human Molt 4/C8 cells, IC50=3.24 μM | ||||
| P388 cells | Cytotoxicity assay | Cytotoxicity evaluated against P388 cells, IC50=0.22 μM | ||||
| MCF-7 | Proliferation assay | Antiproliferative activity in MCF-7 human breast cancer cells, IC50=5.7 μM | ||||
| C6 glioma cell line | Cytotoxicity assay | Cytotoxicity against rat C6 glioma cell line, IC50=12.6 μM | ||||
| HCT116 cells | Cytotoxicity assay | Cytotoxicity against human HCT116 cells, IC50=30.2 μM | ||||
| HCT15 cells | Cytotoxicity assay | Cytotoxicity against human HCT15 cells, IC50=36.3 μM | ||||
| KM12 cells | Cytotoxicity assay | Cytotoxicity against human KM12 cells, IC50=43.7 μM | ||||
| SW620 cells | Cytotoxicity assay | Cytotoxicity against human SW620 cells, IC50=38.9 Μm | ||||
| HCC2998 cells | Cytotoxicity assay | Cytotoxicity against human HCC2998 cells, IC50=41.7 μM | ||||
| SR cells | Cytotoxicity assay | Cytotoxicity against human SR cells, IC50=1.86 μM | ||||
| HSC2 cells | Cytotoxicity assay | Cytotoxicity against HSC2 cells, CC50=35 μM | ||||
| HL60 cells | Cytotoxicity assay | Cytotoxicity against human HL60 cells, CC50=6 μM | ||||
| MOLT3 cells | Function assay | 72 h | Antitumor activity against human MOLT3 cells in presence of Penicillin-G-amidase after 72 hrs by XTT assay, IC50=0.3 μM | |||
| HepG2 cells | Growth inhibition assay | Growth inhibition of human HepG2 cells, GI50=17 μM | ||||
| RT4 cells | Cytotoxicity assay | 96 h | Cytotoxicity against human RT4 cells after 96 hrs by microtiter assay, IC50=14.25 μM | |||
| RT112 cells | Cytotoxicity assay | 96 h | Cytotoxicity against human RT112 cells after 96 hrs by microtiter assay, IC50=4.69 μM | |||
| 5637 cells | Cytotoxicity assay | 96 h | Cytotoxicity against human 5637 cells after 96 hrs by microtiter assay, IC50=0.31 μM | |||
| KYSE70 cells | Cytotoxicity assay | 96 h | Cytotoxicity against human KYSE70 cells after 96 hrs by microtiter assay, IC50=16.16 μM | |||
| KYSE510 | Cytotoxicity assay | 96 h | Cytotoxicity against human KYSE510 cells after 96 hrs by microtiter assay, IC50=8.18 Μm | |||
| KYSE520 cells | Cytotoxicity assay | 96 h | Cytotoxicity against human KYSE520 cells after 96 hrs by microtiter assay, IC50=10.49 μM | |||
| YAPC cells | Cytotoxicity assay | 96 h | Cytotoxicity against human YAPC cells after 96 hrs by microtiter assay, IC50=5.95 μM | |||
| DAN-G cells | Cytotoxicity assay | 96 h | Cytotoxicity against human DAN-G cells after 96 hrs by microtiter assay, IC50=2.65 μM | |||
| SISO cells | Cytotoxicity assay | 96 h | Cytotoxicity against human SISO cells after 96 hrs by microtiter assay, IC50=1 μM | |||
| LCLC-103H cells | Cytotoxicity assay | 96 h | Cytotoxicity against human LCLC-103H cells after 96 hrs by microtiter assay, IC50=4 μM | |||
| MCF7 cells | Cytotoxicity assay | 96 h | Cytotoxicity against human MCF7 cells after 96 hrs by microtiter assay, IC50=3.71 μM | |||
| A427 cells | Cytotoxicity assay | 96 h | Cytotoxicity against human A427 cells after 96 hrs by microtiter assay, IC50=5.13 μM | |||
| Caov3 cells | Cytotoxicity assay | 72 h | Cytotoxicity against human Caov3 cells after 72 hrs by MTT assay | |||
| NSCLC cells | Cytotoxicity assay | 48 hrs | Cytotoxicity against human NSCLC cells assessed as cell growth after 48 hrs by SRB assay, GI50=6.736083 μM | |||
| CNSC cells | Cytotoxicity assay | 48 hrs | Cytotoxicity against human CNSC cells assessed as cell growth after 48 hrs by SRB assay, GI50=7.58578 μM | |||
| mouse FM3A/0 cells | Proliferation assay | 48 hrs | Antiproliferative activity against mouse FM3A/0 cells assessed as inhibition of cell growth after 48 hrs by ZF-Coulter Counting, IC50=3.6 μM | |||
| CEM/0 cells | Proliferation assay | 48 hrs | Antiproliferative activity against human CEM/0 cells assessed as inhibition of cell growth after 48 hrs by ZF-Coulter Counting, IC50=3.5 μM | |||
| HeLa cells | Proliferation assay | 48 hrs | Antiproliferative activity against human HeLa cells assessed as inhibition of cell growth after 48 hrs by ZF-Coulter Counting, IC5=1.9 μM | |||
| INA-6 cells | Cytotoxicity assay | Cytotoxicity against human INA-6 cells assessed as viable fractions using annexin V-FITC/propidium iodide staining by flow cytometry, EC50=2 μM | ||||
| PBMC cells | Cytotoxicity assay | Cytotoxicity against human PBMC cells assessed as viable fractions using annexin V-FITC/propidium iodide staining by flow cytometry, EC50=3 μM | ||||
| SH-SY5Y cells | Cytotoxicity assay | 72 h | Cytotoxicity against human SH-SY5Y cells after 72 hrs by MTT assay, IC50=5.5 μM | |||
| U251 cells | Cytotoxicity assay | 5 days | Cytotoxicity against human U251 cells after 5 days by MTT assay, IC50=3 μM | |||
| A549 cells | Cytotoxicity assay | 5 days | Cytotoxicity against human A549 cells after 5 days by MTT assay, IC50=3 μM | |||
| PANC1 cells | Cytotoxicity assay | 5 days | Cytotoxicity against human PANC1 cells after 5 days by MTT assay, IC50=3 μM | |||
| HT-29 cells | Cytotoxicity assay | 5 days | Cytotoxicity against human HT-29 cells after 5 days by MTT assay, IC50=3 μM | |||
| DLD1 cells | Cytotoxicity assay | 5 days | Cytotoxicity against human DLD1 cells after 5 days by MTT assay, IC50=3 μM | |||
| HeLa cells | Cytotoxicity assay | 4 days | Cytotoxicity against human HeLa cells after 4 days by Coulter counter analysis, IC50=1.9 μM | |||
| FM3A cells | Cytotoxicity assay | 2 days | Cytotoxicity against mouse FM3A cells after 2 days by Coulter counter analysis, IC50=3.6 μM | |||
| HL-60(TB) cells | Function assay | 24 h | Antileukemic activity against human HL-60(TB) cells assessed as inhibition of tumor growth after 24 hrs, IC50=0.38 μM | |||
| SR cells | Function assay | 24 h | Antileukemic activity against human SR cells assessed as inhibition of tumor growth after 24 hrs, IC50=3.24 μM | |||
| L1210 cells | Proliferation assay | 2 days | Antiproliferative activity against mouse L1210 cells assessed as inhibition of cell proliferation incubated for 2 days by Coulter counter based assay, IC50=8.6 μM | |||
| FM3A cells | Proliferation assay | 2 days | Antiproliferative activity against mouse FM3A cells assessed as inhibition of cell proliferation incubated for 2 days by Coulter counter based assay, IC50=3.6 μM | |||
| CEM cells | Proliferation assay | 3 days | Antiproliferative activity against human CEM cells assessed as inhibition of cell proliferation incubated for 3 days by Coulter counter based assay, IC50=3.5 μM | |||
| HeLa cells | Proliferation assay | 4 days | Antiproliferative activity against human HeLa cells assessed as inhibition of cell proliferation incubated for 4 days by Coulter counter based assay, IC50=1.9 μM | |||
| 클릭하여 더 많은 세포주 실험 데이터 보기 | ||||||
화학 정보, 보관 및 안정성
| 분자량 | 305.20 | 화학식 | C13H18Cl2N2O2 |
보관 (수령일로부터) | |
|---|---|---|---|---|---|
| CAS 번호 | 148-82-3 | SDF 다운로드 | 원액 보관 |
|
|
| 동의어 | Alkeran, Sarcolysin, L-PAM | Smiles | C1=CC(=CC=C1CC(C(=O)O)N)N(CCCl)CCCl | ||
용해도
|
In vitro |
DMSO
: 3.5 mg/mL
(11.46 mM)
Water : Insoluble Ethanol : Insoluble |
몰농도 계산기
|
In vivo |
|||||
생체 내 제형 계산기 (투명한 용액)
1단계: 아래 정보 입력 (권장: 실험 중 손실을 고려하여 추가 동물 포함)
2단계: 생체 내 제형 입력 (이것은 계산기일 뿐 제형이 아닙니다. 용해도 섹션에 생체 내 제형이 없는 경우 먼저 당사에 문의하십시오.)
계산 결과:
작업 농도: mg/ml;
DMSO 원액 준비 방법: mg 약물 사전 용해 μL DMSO ( 원액 농도 mg/mL, 농도가 해당 약물 배치의 DMSO 용해도를 초과하는 경우 먼저 당사에 문의하십시오. )
생체 내 제형 준비 방법: 취하다 μL DMSO 원액, 다음 추가μL PEG300, 혼합하고 투명하게 한 다음 추가μL Tween 80, 혼합하고 투명하게 한 다음 추가 μL ddH2O, 혼합하고 투명하게 합니다.
생체 내 제형 준비 방법: 취하다 μL DMSO 원액, 다음 추가 μL 옥수수 기름, 혼합하고 투명하게 합니다.
참고: 1. 다음 용매를 추가하기 전에 액체가 투명한지 확인하십시오.
2. 용매를 순서대로 추가해야 합니다. 다음 용매를 추가하기 전에 이전 추가에서 얻은 용액이 투명한 용액인지 확인해야 합니다. 와동, 초음파 또는 뜨거운 물 중탕과 같은 물리적 방법을 사용하여 용해를 도울 수 있습니다.
작용 메커니즘
| 시험관 내(In vitro) |
골수종 세포주(RPMI 8226)를 30분간 Melphalan (1-phenylalanine-mustard) 펄스에 노출시키면 DNA cross-linking agents의 특징적인 세포 주기 진행 지연이 발생합니다. 이 화합물은 시험관 내 세포에서 DNA, RNA 및 단백질에 결합하는 것으로 나타났습니다. 이는 시험관 내 인간 세포에서 염색체 이상, 자매 염색분체 교환, 미세핵, HPRT 유전자 돌연변이 및 DNA Damage를 유발합니다. 또한 C3H 10T1/2 및 기타 세포의 변형을 유발합니다. 배양된 설치류 세포에서 염색체 이상, 자매 염색분체 교환, 유전자 돌연변이 및 DNA Damage를 유발합니다. 또한, 초파리에서 이수성 및 성염색체 연관 열성 치사 돌연변이를 유발하며, 박테리아에서 돌연변이를 유발합니다.
|
|---|---|
| 생체 내(In vivo) |
Melphalan은 경구, 복강내 및 피부 적용을 통해 생쥐에서, 복강내 주사를 통해 쥐에서, 경구 투여를 통해 원숭이에서 테스트되었습니다. 생쥐에서 이 화합물의 투여는 전위 유두종, 림프육종, 피부 및 폐 종양을 유발했습니다. 쥐에서는 유선 종양 및 복막 육종을 유발했습니다. 원숭이에서의 결과는 결정적이지 않았습니다.
|
참조 |
|
임상시험 정보
(데이터 출처 https://clinicaltrials.gov, 업데이트 날짜 2024-05-22)
| NCT 번호 | 모집 | 조건 | 스폰서/협력자 | 시작일 | 단계 |
|---|---|---|---|---|---|
| NCT06313502 | Not yet recruiting | Plasma Cell Disorder |
University of Arkansas|University of Iowa |
June 2024 | Phase 1 |
| NCT04455139 | Terminated | Eye Cancer Retinoblastoma |
Prof. Beck Popovic Maja|University of Lausanne Hospitals |
November 15 2021 | Phase 2 |
| NCT04945954 | Not yet recruiting | Hematopoietic Stem Cell Transplantation |
Seoul National University Hospital|National Institute of Food and Drug Safety Evaluation (Republic of Korea) |
June 2021 | Not Applicable |
