HDAC inhibitors/activators react with the target HDAC. Histone deacetylases (HDACs) are a family of enzymes that regulate chromatin structure and gene expression by removing acetyl groups from histone proteins. This deacetylation process compacts chromatin, restricting access of transcription factors to DNA and thereby repressing gene transcription. HDAC inhibitors (HDACis), as small-molecule compounds that block HDAC activity, reverse this effect by increasing histone acetylation, leading to chromatin relaxation and the activation of tumor suppressor genes and other functionally important genes. Beyond their role in epigenetics, HDACis also target non-histone proteins, expanding their biological effects across multiple cellular pathways. In recent decades, HDACis have emerged as a pivotal focus in biomedical research, with applications spanning cancer therapy, neurodegenerative disease, and autoimmune disorders.
HDAC 억제제/활성제 (HDAC Inhibitors/Activators)
기타 DNA Damage/DNA Repair 억제제
| Cat.No. | 제품명 | 정보 | 제품 사용 인용 | 제품 검증 |
|---|---|---|---|---|
| S2170 | ITF-2357 (Givinostat) Hydrochloride Monohydrate | Givinostat (ITF2357)는 옥수수 HD2, HD1B 및 HD1A에 대한 강력한 HDAC 억제제로, 세포 유리 분석에서 IC50은 각각 10 nM, 7.5 nM 및 16 nM입니다. 2상. |
|

|
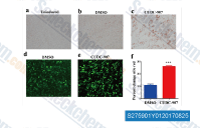
| S2759 | Fimepinostat (CUDC-907) | Fimepinostat (CUDC-907)은 PI3Kα 및 HDAC1/2/3/10을 표적으로 하는 이중 PI3K 및 HDAC 억제제로, IC50 값은 각각 19 nM 및 1.7 nM/5 nM/1.8 nM/2.8 nM입니다. 이 화합물은 유방암 세포에서 세포 주기 정지 및 apoptosis를 유도합니다. 1상. |
|
|
| S1045 | Trichostatin A (TSA) | TSA (Trichostatin A)는 무세포 분석에서 IC50이 약 1.8 nM인 HDAC 억제제입니다. |
|
|
| S1053 | Entinostat (MS-275) | Entinostat (MS-275, SNDX-275)는 세포 없는 분석에서 HDAC1 및 HDAC3을 0.51 μM 및 1.7 μM의 IC50으로 강력하게 억제하며, 이는 HDAC 4, 6, 8, 10과 비교된다. 이 화합물은 autophagy 및 apoptosis를 유도한다. 3상. |
|
|
| S7229 | RGFP966 | RGFP966은 무세포 분석에서 IC50 0.08 μM인 HDAC3 억제제로, 다른 HDAC에 비해 200배 이상의 선택성을 나타냅니다. |
|

|
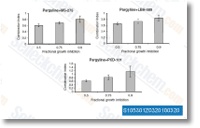
| S1030 | Panobinostat (LBH589) | 파노비노스타트 (LBH589, NVP-LBH589)는 무세포 분석에서 IC50 5nM을 나타내는 새로운 광범위 HDAC 억제제입니다. 이는 autophagy와 apoptosis를 유도하며, 생체 내에서 HIV 잠복기를 효과적으로 방해합니다. 3상. |
|
|
| S1085 | Belinostat (PXD101) | Belinostat은 세포 없는 분석에서 IC50가 27nM인 새로운 HDAC 억제제로, 시스플라틴 내성 종양에서 활성이 입증되었습니다. Belinostat (PXD101)은 autophagy를 유도합니다. |
|

|
| S1047 | Vorinostat (SAHA) | Vorinostat (SAHA)는 HDAC 억제제로, 무세포 분석에서 IC50이 약 10 nM이며 HPV-18 DNA의 생산적인 증폭을 저해합니다. |
|

|
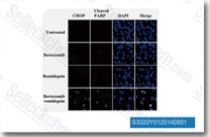
| S3020 | Romidepsin (FK228) | Romidepsin (FK228, Depsipeptide, FR 901228, NSC 630176)은 무세포 분석에서 각각 36 nM 및 47 nM의 IC50을 갖는 강력한 HDAC1 및 HDAC2 억제제입니다. 이 화합물은 신경모세포종 종양 세포의 성장을 조절하고 apoptosis를 유도합니다. |
|
|
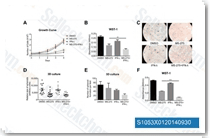
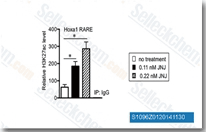
| S1096 | Quisinostat (JNJ-26481585) Dihydrochloride | Quisinostat (JNJ-26481585) 2HCl은 세포 유리 분석에서 0.11 nM의 IC50으로 HDAC1에 대해 가장 높은 효능을 보이는 새로운 2세대 HDAC 억제제이며, HDACs 2, 4, 10 및 11에 대해 적당한 효능을 보이고; HDACs 3, 5, 8 및 9에 대해 30배 이상의 선택성을 보이며 HDACs 6 및 7에 대해 가장 낮은 효능을 보입니다. 2상. |
|
|
Classification of HDACs and HDAC Inhibitors
To understand HDACis, it is first critical to categorize their targets: HDACs are divided into four classes based on sequence homology and cofactor requirements. Class I HDACs (HDAC1, 2, 3, 8) are primarily localized in the nucleus and are ubiquitously expressed, playing essential roles in cell cycle regulation and proliferation. Class II HDACs (subdivided into IIa: HDAC4, 5, 7, 9; IIb: HDAC6, 10) shuttle between the nucleus and cytoplasm, with Class IIa involved in developmental signaling and Class IIb regulating cytoskeletal dynamics and protein degradation. Class III HDACs, also called sirtuins (SIRT1–7), depend on NAD+ as a cofactor and are implicated in metabolism, aging, and stress responses. Class IV HDAC (HDAC11) is the smallest family member, with functions in immune cell activation and lipid metabolism. HDACis are correspondingly classified based on their chemical structure and HDAC class selectivity:
1.1 Pan-HDACis:
Inhibit multiple HDAC classes, particularly Class I and II. Examples include vorinostat (SAHA) and trichostatin A (TSA). Vorinostat, the first FDA-approved HDACi (2006), targets Class I (HDAC1, 2, 3) and Class IIb (HDAC6) and is used to treat cutaneous T-cell lymphoma (CTCL).
1.2 Class I-Selective HDACis:
Preferentially inhibit Class I HDACs, reducing off-target effects on Class II enzymes. Romidepsin, a cyclic peptide HDACi, selectively targets HDAC1 and 2 and is approved for relapsed CTCL and peripheral T-cell lymphoma (PTCL).
1.3 Class II-Selective HDACis:
Focus on Class II HDACs, with potential applications in neurodegeneration and cardiovascular disease. For example, MC1568 inhibits Class IIa HDACs (HDAC4, 5, 7) and has shown promise in preclinical models of Huntington’s disease.
1.4 Class III HDAC (Sirtuin) Modulators:
Unlike other HDACis, sirtuin-targeting compounds often act as activators (e.g., resveratrol, which activates SIRT1) due to the unique NAD+-dependent mechanism of sirtuins. These modulators are studied for their roles in aging and metabolic disorders.
2. Mechanisms of Action: Beyond Histone Acetylation
The canonical mechanism of HDACis involves histone acetylation, but their biological effects extend far beyond this epigenetic regulation, driven by the modulation of non-histone proteins.
2.1 Histone-Mediated Epigenetic Regulation
HDACs remove acetyl groups from the ε-amino groups of lysine residues on histone tails (e.g., H3K9, H3K14, H4K8). This deacetylation increases the positive charge of histones, strengthening their electrostatic interaction with negatively charged DNA and forming a condensed chromatin structure (heterochromatin) that blocks transcription. HDACis bind to the catalytic site of HDACs, preventing deacetylation and accumulating acetylated histones. This relaxed chromatin (euchromatin) allows transcription factors, such as p53 and E2F, to bind to promoter regions, activating the expression of tumor suppressor genes (e.g., p21 Bax) and genes involved in cell cycle arrest and apoptosis. In cancer cells, this reactivation reverses the oncogenic silencing of critical pathways, suppressing proliferation and inducing cell death.
2.2 Regulation of Non-Histone Substrates
Non-histone proteins constitute a large fraction of HDAC substrates, and their acetylation status controls protein function, localization, and stability. HDACis modulate key non-histone targets, including:
p53: A master tumor suppressor. Acetylation of p53 (at lysine residues 373 and 382) by HDAC inhibition enhances its stability and DNA-binding activity, promoting cell cycle arrest and apoptosis in cancer cells.
NF-κB: A transcription factor central to inflammation and immune responses. HDACs deacetylate the p65 subunit of NF-κB, enhancing its transcriptional activity. HDACis block this deacetylation, inhibiting NF-κB-mediated expression of pro-inflammatory cytokines (e.g., TNF-α, IL-6) and reducing tumor cell survival.
α-Tubulin: A component of the cytoskeleton. HDAC6 (a Class IIb HDAC) deacetylates α-tubulin, regulating microtubule dynamics. HDACis targeting HDAC6 increase α-tubulin acetylation, disrupting mitosis and inhibiting cancer cell migration and invasion.
These non-histone effects expand the therapeutic potential of HDACis beyond cancer, as seen in their ability to reduce inflammation in autoimmune diseases and protect neurons in neurodegenerative disorders.
3. Key Research Advances in HDAC Inhibitor Development
Recent research has advanced HDACi development in three major directions: optimizing efficacy in cancer therapy, exploring applications in non-oncological diseases, and improving selectivity to reduce side effects.
3.1 Cancer Therapy: From Monotherapy to Combination Strategies
While single-agent HDACis have shown efficacy in hematological malignancies (e.g., CTCL, PTCL), their performance in solid tumors has been limited due to tumor heterogeneity and drug resistance. To address this, researchers have focused on combination therapies that synergize with HDACis:
Immune Checkpoint Inhibitors: HDAC is modulate the tumor microenvironment by increasing the expression of tumor-associated antigens (TAAs) and MHC class I molecules, enhancing T-cell recognition of cancer cells. Preclinical studies have shown that combining vorinostat with anti-PD-1 antibodies (e.g., pembrolizumab) improves T-cell infiltration and tumor regression in melanoma and non-small cell lung cancer (NSCLC). A 2023 clinical trial (NCT03123096) reported that the combination of romidepsin and (e.g., nivolumab) achieved a 35% objective response rate in relapsed PTCL, significantly higher than romidepsin monotherapy (15%).
Targeted Therapies: HDACis synergize with inhibitors of oncogenic pathways, such as the PI3K/Akt/mTOR pathway. In breast cancer models, combining the Class I HDACi entinostat with the PI3K inhibitor (e.g., alpelisib) enhances apoptosis by blocking mTOR-mediated survival signals. Similarly, in glioblastoma, HDACis reverse the resistance to EGFR inhibitors by reactivating silenced tumor suppressor genes (e.g., PTEN).
3.2 Non-Oncological Applications: Neurodegeneration and Autoimmunity
HDACis have gained traction in non-cancer research, particularly in neurodegenerative diseases where epigenetic dysregulation contributes to pathogenesis:
3.2.1 Alzheimer’s Disease (AD): HDACis increase the acetylation of histones and tau protein (a key component of neurofibrillary tangles in AD). Preclinical studies with vorinostat and panobinostat have shown reduced tau hyperphosphorylation and improved cognitive function in AD mouse models. A 2022 study in Nature Communications reported that a Class IIa HDACi (HDAC4/5 inhibitor) enhances the clearance of amyloid-beta (Aβ) plaques by activating microglia, a critical step in reducing neuroinflammation in AD.
3.2.2 Autoimmune Diseases: HDACis suppress immune cell activation by inhibiting NF-κB and reducing cytokine production. In rheumatoid arthritis (RA), HDACis (e.g., givinostat) have been tested in phase II trials, showing reduced joint inflammation and improved disease activity scores by inhibiting the proliferation of synovial fibroblasts and the production of IL-6 and TNF-α.
