연구용
Brefeldin A (BFA chemical) 단백질 수송 억제제
제품 번호S7046
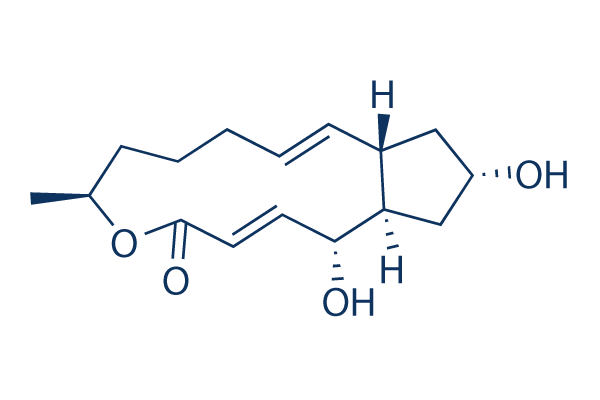
화학 구조
분자량: 280.36
품질 관리
세포 배양, 처리 및 작업 농도
| 세포주 | 분석 유형 | 농도 | 배양 시간 | 제형 | 활성 설명 | PMID |
|---|---|---|---|---|---|---|
| PC12 | Function Assay | 2 μM | 1 h | inhibits the L-DOPA (20 μM)-induced transient ERK1/2 phosphorylation | 26363191 | |
| C2C12 | Function Assay | 1 μg/ml | 1 h | abolishes cytokine release from C2C12 myotubes | 26291279 | |
| MEFs WT | Function Assay | 5 μM | 20 min | causes resident enzymes such as NAGT-GFP, to diffuse back to the ER | 26196023 | |
| MEFs VAMP7 KO | Function Assay | 5 μM | 20 min | causes resident enzymes such as NAGT-GFP, to diffuse back to the ER | 26196023 | |
| SMCs | Function Assay | 10 µg/ml | 0-12 h | DMSO | shows a trend towards a higher concentration of the ER/SR network in the perinuclear area | 26172080 |
| SMCs | Function Assay | 10 µg/ml | 0-12 h | DMSO | causes a transient Ca2+ release from the ER/SR | 26172080 |
| HEMC-1 | Function Assay | 0.1 µg/ml | 24 h | causes a higher inhibitory effect on exocytosis than nocodazole | 25972759 | |
| HUVEC | Function Assay | 10 μM | 1 h | DMSO | abolishes hypoxia-induced release of ATP from apical and basolateral surfaces | 25956988 |
| HUVEC | Function Assay | 10 μM | 1 h | DMSO | increases the number and intensity of fluorescent areas especially in perinuclear space | 25956988 |
| Caco-2 | Function Assay | 2.5 μM | 30 min | attenuates the TGF-β1-mediated increase in SERT function | 25954931 | |
| NRK | Function Assay | 200 ng/ml | 4 h | DMSO | rescues mitotic progression | 25948586 |
| HeLa | Function Assay | 200 ng/ml | 3 h | DMSO | induces the artificial break-up of the Golgi complex | 25948586 |
| COS | Function Assay | 1 μg/ml | 3 h | completely disperses the AP-1 signal | 25915900 | |
| DF1 | Function Assay | 1 μM | 48 h | DMSO | disperses the exogenous CSGalNAcT2 protein | 25807054 |
| nHDFs | Function Assay | 1 μM | 2 h | prevents the assembly of cytosolic coat proteins onto Golgi membranes | 25772616 | |
| FRT | Function Assay | 5 μg/ml | 2 h | blocks trafficking through the Golgi complex by inhibiting ER-to-Golgi transport | 25767115 | |
| FRT | Function Assay | 5 μg/ml | 2 h | prevents the increase in cleaved α subunits when [Na+]i was reduced | 25767115 | |
| HepG2 | Function Assay | 1 µM | 24 h | DMSO | decreases the level of PXR mRNA | 25616597 |
| SMCs | Function Assay | 1μg/mL | 3 h | accumulates CNPY2 protein in the ER compartment and no longer co-localized with the Golgi marker | 25589425 | |
| OB-6 | Apoptosis Assay | 2.7 μM | 48 h | induces apoptosis | 25532480 | |
| iPSC-CMs | Function Assay | 500 ng/ml | 48 h | increases the intensity of the higher mobility LAMPs at the cost of the lower mobility species | 25488666 | |
| SP-Nluc | Function Assay | 5 mg/mL | 6 h | DMSO | causes an increase in reporter activity in the parasite | 25392998 |
| PEXEL-Nluc | Function Assay | 5 mg/mL | 6 h | DMSO | causes an increase in reporter activity in the parasite | 25392998 |
| H1299 | Function Assay | 10 μg/ml | 24 h | induces autophagy | 25388970 | |
| MDA-MB-231 | Cell Viability Assay | 0–50 μg/mL | 48 h | EC50 = 0.016 µg/mL | 25356567 | |
| MDA-MB-231 | Apoptosis Assay | 0.1 μg/mL | 4 h | induces apoptosis | 25356567 | |
| MDA-MB-231 | Growth Inhibition Assay | 0.01/0.05 μg/mL | 24 h | increases the fraction of sub-G1 cell debris | 25356567 | |
| MDA-MB-231 | Apoptosis Assay | 0.05–1 μg/mL | 24 h | induces PARP (poly ADP-ribose polymerase-1) cleavage | 25356567 | |
| MDA-MB-231 | Function Assay | 0–50 μg/mL | 24 h | inhibits the formation of 3D and 2D colonies | 25356567 | |
| A172 | Function Assay | 10 μg/ml | 4 h | DMSO | results in the retrograde transport of fluorescent granules | 25239507 |
| KMS-6 | Function Assay | 1 μM | 24 h | exhibits half the secretion of galanin-LI as did the control | 25229126 | |
| MEC | Function Assay | 1 μM | 1.5 h | causes a dramatic decrease in the surface VEGFR2 | 25228815 | |
| HEK293/hERG | Function Assay | 10 μM | 1 h | results in a time-dependent reduction mature hERG protein | 25218469 | |
| RBE4 | Apoptosis Assay | 2 μM | 3–24 h | induces apoptosis time dependently | 25128025 | |
| RBE4 | Function Assay | 2 μM | 3–24 h | increases the XBP1 protein levels after 3 and 6 h of treatment | 25128025 | |
| RBE4 | Function Assay | 2 μM | 3–24 h | increases active caspase-12 in a time-dependent manner | 25128025 | |
| RBE4 | Function Assay | 2 μM | 3–24 h | increases the levels of ROS time-dependently | 25128025 | |
| RBE4 | Function Assay | 2 μM | 3–24 h | induces a delayed depletion of the ER Ca2+ content at 6 h of incubation significantly | 25128025 | |
| RBE4 | Function Assay | 2 μM | 3–24 h | induces an overload of Ca2+ in the mitochondria in the first 6 h of incubation (p < 0.001) but Ca2+ levels in this organelle decreased after 12 h of incubation | 25128025 | |
| Huh-7 | Function Assay | 1μg/mL | 3–24 h | increases the level of APE1 in a time-dependent manner | 25026174 | |
| HepG2 | Function Assay | 1μg/mL | 3–24 h | increases the level of APE1 in a time-dependent manner | 25026174 | |
| H838-LKB1 | Function Assay | 30 ng/ml | 12/18 h | increases the protein levels of BiP | 25011082 | |
| H838-KDLKB1 | Function Assay | 30 ng/ml | 12/18 h | increases the protein levels of BiP | 25011082 | |
| H838-KDLKB1 | Function Assay | 30 ng/ml | 12/18 h | increases the levels of phosphorylated eIF2α (phospho-eIF2α) | 25011082 | |
| 3T3-L1 | Function Assay | 5 μg/ml | 30 min | mimics the effects of insulin and causes robust phosphorylation of Akt (Ser 473) and phosphorylation of AS160 (Thr 642 and Ser 588) | 24843827 | |
| 3T3-L1 | Function Assay | 5 μg/ml | 30 min | recapitulates insulin action with respect to regulating Akt activity and AS160 phosphorylation | 24843827 | |
| 3T3-L1 | Function Assay | 5 μg/ml | 30 min | causes reversible redistribution of GLUT4 | 24843827 | |
| 3T3-L1 | Function Assay | 5 μg/ml | 1 h | causes redistribution of GLUT4 but not increase in glucose uptake | 24843827 | |
| 3T3-L1 | Function Assay | 5 μg/ml | 1 h | causes phosphorylation of the FoxO1 transcription factor | 24843827 | |
| HeLa | Function Assay | 5 μg/ml | 3 h | causes nuclear exclusion of the FoxO1 transcription factor and decreases transcription of FoxO1-regulated genes | 24843827 | |
| HEK293 | Function Assay | 5 μg/ml | 12 h | abolishes CMA-induced CRELD2 secretion | 24687431 | |
| COS-1 | Function Assay | 5 µg/ml | 24 h | restricts localization of NB in the perinuclear region | 24671751 | |
| PRP | Function Assay | 10 μM | abrogates SDF-1α-mediated CXCR7 externalization | 24668750 | ||
| RAW264.7 | Apoptosis Assay | 4 μM | 48 h | attenuates the inhibition of ox-LDL-induced apoptosis and the facilitation of cholesterol efflux by Ac-hE-18A-NH2 | 24639032 | |
| MDMs | Apoptosis Assay | 10 μg/ml | 12/15 h | induces apoptosis | 24556695 | |
| PMHs | Function Assay | 10–20 μg/ml | 24 h | DMSO | induced ER stress | 24407242 |
| PMHs | Apoptosis Assay | 10–20 μg/ml | 24 h | DMSO | increases cell death | 24407242 |
| HEK293/tau | Function Assay | 5 μM | 1/2/4 h | induces Golgi fragmentation | 24368089 | |
| HEK293/tau | Function Assay | 5 μM | 3 h | induces tau hyperphosphorylation | 24368089 | |
| ADF | Function Assay | 10 μM | 16 h | inhibits the ZnCl2-induced translocation of CRT | 24228232 | |
| U373 | Function Assay | 10 μM | 16 h | inhibits the ZnCl2-induced translocation of CRT | 24228232 | |
| RKO-HIPK2i | Function Assay | 10 μM | 16 h | inhibits the ZnCl2-induced translocation of CRT | 24228232 | |
| ADF | Function Assay | 10 μM | 6 h | impairs the DC activation | 24228232 | |
| Huh7 | Function Assay | 5 μg/ml | 4 h | abolishes the secretion of intracellular ApoB | 24100140 | |
| Huh7 | Function Assay | 5 μg/ml | 1 h | causes a significant increase in ApoB-crescents | 24100140 | |
| Huh7 | Function Assay | 5–10 ng/ml | 12 h | increases ApoB-crescents without inhibiting secretion | 24100140 | |
| BAECs | Function Assay | 5 μg/ml | 0-4 h | induces the rapid dephosphorylation of eNOS at Ser1179 | 24085225 | |
| Macrophages | Function Assay | 71 µM | 6 h | inhibits lunasin internalization | 24039740 | |
| Colo 205 | Growth Inhibition Assay | 0-5 μg/mL | 48 h | inhibits cell growth in suspension cultures with an estimated IC50 of ~15 ng/mL | 23973996 | |
| Colo 205 | Function Assay | 0.012-0.025 μg/mL | 14 d | reduces the clonogenicity of Colo 205 CSCs | 23973996 | |
| Colo 205 | Apoptosis Assay | 0.1 μg/mL | 0-24 h | induces apoptosis of Colo 205 cells in suspension cultures | 23973996 | |
| Colo 205 | Function Assay | 0.015 μg/mL | 24 h | induces the expression of ER stress-related genes | 23973996 | |
| Colo 205 | Function Assay | 0.015 μg/mL | 24 h | inhibits the activity of MMPs | 23973996 | |
| IBRS2 | Function Assay | 5 μg/ml | 0.5 h | DMSO | disrupts the ERGIC and Golgi | 23963534 |
| IBRS2 | Function Assay | 5 μg/ml | 0.5 h | DMSO | enhances FMDV infection | 23963534 |
| HeLa | Function Assay | 2 μM | 2 h | attenuates the TNF-induced secretion of IL-15 | 23950892 | |
| HFS | Function Assay | 0-1 μg/ml | 24 h | GLTP expression reaches a plateau at concentrations as low as 0.01 µg/ml | 23894633 | |
| HFS | Function Assay | 0.01 µg/ml | 24 h | increases the expression of glycosphingolipid synthase genes at 6 h | 23894633 | |
| OVCAR-3 | Growth Inhibition Assay | 1–15 μM | 24 h | induces a loss of cell viability dose dependently | 23826964 | |
| OVCAR-3 | Function Assay | 1–15 μM | 24 h | induces nuclear damage | 23826964 | |
| OVCAR-3 | Apoptosis Assay | 1-10 μM | 4 h | induces the activation of apoptosis-related proteins | 23826964 | |
| OVCAR-3 | Apoptosis Assay | 10 μM | 24 h | induces activation of caspases | 23826964 | |
| OVCAR-3 | Function Assay | 1–10 μM | 24 h | induces disruption of the mitochondrial transmembrane potential | 23826964 | |
| OVCAR-3 | Function Assay | 1–10 μM | 24 h | induces formation of reactive oxygen species | 23826964 | |
| OVCAR-3 | Function Assay | 1–10 μM | 24 h | inhibits cell adhesion and migration | 23826964 | |
| MKN45 | Growth Inhibition Assay | IC50<0.001 μg/ml | 23793342 | |||
| LOVO | Growth Inhibition Assay | IC50=0.12 μg/ml | 23793342 | |||
| A549 | Growth Inhibition Assay | IC50=0.04 μg/ml | 23793342 | |||
| MDA-MB-435 | Growth Inhibition Assay | IC50<0.001 μg/ml | 23793342 | |||
| HepG2 | Growth Inhibition Assay | IC50<0.001 μg/ml | 23793342 | |||
| HL-60 | Growth Inhibition Assay | IC50<0.001 μg/ml | 23793342 | |||
| neural precursor cells | Function assay | Inhibition of neurosphere proliferation of mouse neural precursor cells by MTT assay | 17417631 | |||
| HeLa | Function assay | 100 uM | 2 hrs | Dispersion of cis golgi marker betaCoP in human HeLa cells at 100 uM for 2 hrs | 17563369 | |
| HeLa | Function assay | 100 uM | 2 hrs | Dispersion of cis golgi marker KDEL in human HeLa cells at 100 uM for 2 hrs | 17563369 | |
| Vero | Function assay | 10 ug/ml | 5 mins | Inhibition of Arf1 in african green monkey Vero cells assessed as rapid AP-1 dispersal from golgi membranes at 10 ug/ml after 5 mins by immunofluorescence method | 19182783 | |
| Vero | Function assay | 10 ug/ml | 5 mins | Inhibition of Arf1 in african green monkey Vero cells assessed as rapid GGA3 dispersal from trans golgi network at 10 ug/ml after 5 mins by immunofluorescence method | 19182783 | |
| Vero | Function assay | 10 uM | 1 hr | Inhibition of GBF1 QNV deleted mutant in african green monkey Vero cells assessed as effect on change in golgi morphology at 10 uM after 1 hr by immunofluorescence method | 19182783 | |
| Vero | Function assay | 10 uM | 1 hr | Inhibition of GBF1 QNV to AAA mutant in african green monkey Vero cells assessed as effect on change in golgi morphology at 10 uM after 1 hr by immunofluorescence method | 19182783 | |
| Vero | Function assay | 10 ug/ml | 1 hr | Inhibition of Arf1 in african green monkey Vero cells assessed as decrease in Arf1-GTP levels at 10 ug/ml after 1 hr | 19182783 | |
| Vero | Function assay | 10 uM | Inhibition of GBF1 in african green monkey Vero cells assessed as inhibition of StxB-SS retrogade transport from endosomes to TGN at 10 uM by immunofluorescence method | 19182783 | ||
| Vero | Function assay | 10 uM | 1 hr | Inhibition of GBF1 in african green monkey Vero cells assessed as punctate and diffuse distribution of medial-Golgi marker giantin from TGN at 10 uM after 1 hr by immunofluorescence method | 19182783 | |
| Vero | Function assay | 10 ug/ml | 1 hr | Inhibition of Arf1 in african green monkey Vero cells assessed as punctate and diffuse distribution of medial-Golgi marker giantin at 10 ug/ml after 1 hr by immunofluorescence method | 19182783 | |
| Vero | Function assay | 10 ug/ml | 5 mins | Inhibition of Arf1 in african green monkey Vero cells assessed as rapid COPI redistribution from golgi at 10 ug/ml after 5 mins by immunofluorescence method | 19182783 | |
| Vero | Function assay | 10 ug/ml | 1 hr | Inhibition of Arf1 in african green monkey Vero cells assessed as tubule formation from trans golgi network and endosomes before its dispersal at 10 ug/ml after 1 hr by immunofluorescence method | 19182783 | |
| Vero | Function assay | 10 ug/ml | 1 hr | Inhibition of Arf1 in african green monkey Vero cells assessed as giantin positive punctate structures in contact with Sec31-positive ER exit site at 10 ug/ml after 1 hr by immunofluorescence method | 19182783 | |
| Vero | Function assay | 10 ug/ml | Inhibition of Arf1 in african green monkey Vero cells assessed as inhibition of StxB-SS retrogade transport from endosomes to TGN at 10 ug/ml by immunofluorescence method | 19182783 | ||
| Vero | Function assay | 10 uM | 1 hr | Induction of GBF1 in african green monkey Vero cells assessed as punctate and diffuse distribution of cis-Golgi marker GM130 from TGN at 10 uM after 1 hr by immunofluorescence method | 19182783 | |
| Vero | Function assay | 10 ug/ml | 1 hr | Inhibition of Arf1 in african green monkey Vero cells assessed as punctate and diffuse distribution of cis-Golgi marker GM130 at 10 ug/ml after 1 hr by immunofluorescence method | 19182783 | |
| NRK | Function assay | 7 uM | 60 mins | Golgi-disturbing activity in golgi apparatus of rat NRK cells assessed as fusion of golgi membrane fusion with endoplasmic reticulum at 7 uM after 60 mins by Hoechst 3342 staining-based immunofluorescence microscopy | 20189813 | |
| NCI60 | Cytostatic assay | Cytostatic activity against human NCI60 cells by SRB assay, GI50=0.0206μM. | 23805957 | |||
| NCI60 | Cytostatic assay | Cytostatic activity against human NCI60 cells by SRB assay, TGI=3.48μM. | 23805957 | |||
| HeLa R19 | Antiviral assay | 0.5 uM | 7 hrs | Antiviral activity against Coxsackievirus B3 infected in human HeLa R19 cells assessed as inhibition of viral replication at 0.5 uM after 7 hrs by luciferase reporter gene assay | 23805957 | |
| HeLa | Function assay | 5 uM | 30 to 60 mins | Induction of golgi apparatus disassembly in human HeLa cells at 5 uM after 30 to 60 mins by confocal microscopic analysis | 23805957 | |
| Arabidopsis thaliana root cells | Function assay | 90 uM | 30 mins | Induction of morphological changes of golgi apparatus in Arabidopsis thaliana root cells expressing ST-YFP/VHAa1-RFP at 90 uM after 30 mins by confocal laser scanning microscopic analysis | 23805957 | |
| HeLa R19 | Antiviral assay | 5 to 50 uM | 7 hrs | Antiviral activity against Coxsackievirus B3 infected in human HeLa R19 cells assessed as inhibition of viral replication at 5 to 50 uM after 7 hrs by luciferase reporter gene assay | 23805957 | |
| PC3 | Function assay | 50 nM | 72 hrs | Potentiation of 3 nM docetaxel-induced cytotoxicity against human PC3 cells assessed as decrease in cell viability at 50 nM after 72 hrs by trypan blue exclusion assay | 28462831 | |
| L02 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human L02 cells assessed as reduction in cell viability after 72 hrs by MTT assay, IC50<0.0004μM. | 28494251 | ||
| PC3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human PC3 cells after 72 hrs by MTT assay, IC50=0.068μM. | 28494251 | ||
| HT-29 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HT-29 cells after 72 hrs by MTT assay, IC50=0.16μM. | 28494251 | ||
| HepG2 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HepG2 cells after 72 hrs by MTT assay, IC50=0.35μM. | 28494251 | ||
| LO2 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human LO2 cells after 72 hrs by MTT assay, IC50<0.001μM. | 29524728 | ||
| Bel7402 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human Bel7402 cells after 72 hrs by MTT assay, IC50=0.024μM. | 29524728 | ||
| HL60 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HL60 cells after 72 hrs by MTT assay, IC50=0.025μM. | 29524728 | ||
| PC3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human PC3 cells after 72 hrs by MTT assay, IC50=0.068μM. | 29524728 | ||
| Bel7402/5-FU | Antiproliferative assay | 72 hrs | Antiproliferative activity against human Bel7402/5-FU cells after 72 hrs by MTT assay, IC50=0.82μM. | 29524728 | ||
| HeLa | Function assay | 18 uM | 3 hrs | Inhibition of alkaline phosphatase secretion in human HeLa cells at 18 uM incubated for 3 hrs | 31421965 | |
| VERO-E6 | Function assay | 48 hrs | Determination of IC50 values for inhibition of SARS-CoV-2 induced cytotoxicity of VERO-E6 cells after 48 hours exposure to 0.01 MOI SARS CoV-2 virus by high content imaging, IC50=0.02μM. | ChEMBL | ||
| VERO-E6 | Function assay | 48 hrs | Toxicity CC50 against VERO-E6 cells determined at 48 hours by high content imaging (same conditions as 2_LEY without exposure to 0.01 MOI SARS CoV-2 virus), CC50=0.06μM. | ChEMBL | ||
| 클릭하여 더 많은 세포주 실험 데이터 보기 | ||||||
화학 정보, 보관 및 안정성
| 분자량 | 280.36 | 화학식 | C16H24O4 |
보관 (수령일로부터) | |
|---|---|---|---|---|---|
| CAS 번호 | 20350-15-6 | SDF 다운로드 | 원액 보관 |
|
|
| 동의어 | Cyanein, Decumbin | Smiles | CC1CCCC=CC2CC(CC2C(C=CC(=O)O1)O)O | ||
용해도
|
In vitro |
DMSO
: 56 mg/mL
(199.74 mM)
Water : Insoluble Ethanol : Insoluble |
몰농도 계산기
|
In vivo |
|||||
생체 내 제형 계산기 (투명한 용액)
1단계: 아래 정보 입력 (권장: 실험 중 손실을 고려하여 추가 동물 포함)
2단계: 생체 내 제형 입력 (이것은 계산기일 뿐 제형이 아닙니다. 용해도 섹션에 생체 내 제형이 없는 경우 먼저 당사에 문의하십시오.)
계산 결과:
작업 농도: mg/ml;
DMSO 원액 준비 방법: mg 약물 사전 용해 μL DMSO ( 원액 농도 mg/mL, 농도가 해당 약물 배치의 DMSO 용해도를 초과하는 경우 먼저 당사에 문의하십시오. )
생체 내 제형 준비 방법: 취하다 μL DMSO 원액, 다음 추가μL PEG300, 혼합하고 투명하게 한 다음 추가μL Tween 80, 혼합하고 투명하게 한 다음 추가 μL ddH2O, 혼합하고 투명하게 합니다.
생체 내 제형 준비 방법: 취하다 μL DMSO 원액, 다음 추가 μL 옥수수 기름, 혼합하고 투명하게 합니다.
참고: 1. 다음 용매를 추가하기 전에 액체가 투명한지 확인하십시오.
2. 용매를 순서대로 추가해야 합니다. 다음 용매를 추가하기 전에 이전 추가에서 얻은 용액이 투명한 용액인지 확인해야 합니다. 와동, 초음파 또는 뜨거운 물 중탕과 같은 물리적 방법을 사용하여 용해를 도울 수 있습니다.
작용 메커니즘
| Targets/IC50/Ki |
ATPase (HCT 116)
0.2 μM
|
|---|---|
| 시험관 내(In vitro) |
Brefeldin A (BFA)는 소포체와 골지체 사이의 전방 수송을 차단하여 막 단백질의 분포를 손상시키는 곰팡이 대사물입니다. HCT 116 인간 결장암 세포를 이 화합물로 처리하면 세포 분화를 나타내는 형태학적 변화가 관찰됩니다. 이는 주로 종양 세포에서 분화와 apoptosis를 유도함으로써 세포독성 효과를 발휘합니다. 20 μg/mL BFA로 6시간 동안 스트립을 처리하면 10mM 인도메타신 및 30 μM L-NOARG 존재하에 브라디키닌에 의해 유도된 이완이 완전히 사라집니다. 20 μg/mL의 이 화합물로 처리하면 1 nM에서 1 mM 사이의 농도 범위에서 브라디키닌에 의해 유도된 [Ca2+]i 및 장력 감소가 상당히 사라집니다. 이는 브라디키닌 또는 물질 P에 의해 유도된 내피 세포의 [Ca2+]i 상승에 영향을 미치지 않습니다. 곰팡이 대사물을 첨가해도 myr-rARF1에 대한 자발적인 인지질 의존적 GTPS 결합에는 영향을 미치지 않지만, 망막 등장액(RIE) 촉매 교환은 완전히 사라지며, 반최대 억제는 2 μM입니다. 이는 다양한 막 트래픽 경로를 방지하고 골지체 막 또는 뇌 세포질에 존재하는 ADP-리보실화 인자 특이적 구아닌 뉴클레오타이드 교환 활성을 억제합니다. 이 화합물에 의한 완전한 방지는 망막 추출물이 ARF 특이적 구아닌 뉴클레오타이드 교환 인자를 포함하고 있음을 강력히 시사합니다. 두 ADP-리보실화 인자(ARF)로부터 망막 등장액(RIE) 촉매 GTPS 방출은 300 μM에서도 BFA에 의해 부분적으로만 억제됩니다. 이는 골지체와 ER의 융합을 유도하고 CERT 억제제 HPA-12의 억제 효과를 제거합니다. 골지체와 ER의 융합을 유도하는 이 화합물로 처리하면 리모노이드 유도 스핑고미엘린 생합성 방지를 회복시킵니다. CHO 세포를 처리하면 스핑고미엘린 합성이 2~3배 증가합니다. B-CLL 세포 외에도 BFA는 다발성 골수종 (U266, NCI-H929), Jurkat, HeLa, 백혈병 (HL60, K562, BJAB), 결장 (HT-29) 및 전립선, 그리고 샘낭성 육종 세포에서 apoptosis를 유발하는 것으로 보고되었습니다. 이 화합물 25 ng/mL를 투여하면 HF4.9 및 HF28RA 세포의 성장을 완전히 차단하지만, HF1A3 세포에서 동일한 효과를 얻기 위해서는 더 높은 용량 (75 ng/mL)이 필요합니다. 세포 증식은 24시간 이내에 용량 의존적으로 억제되며, 세포주에 따라 50-75 ng/mL에서 3H-티미딘 통합이 거의 완전히 중단됩니다 (HF1A3, HF4.9, HF28RA 세포의 경우 50 ng/ml에서 26%, 76%, 87% 억제, 75 ng/mL에서 75%, 87%, 92% 억제). BFA 유도 세포 사멸은 YO-PRO 1/PI 분석을 사용하여 용량 의존적으로 발생합니다. 이는 HDR(homology-directed repair) 효율을 향상시킬 수 있으며 CRISPR-mediated HDR의 강화제입니다. |
| 생체 내(In vivo) |
Brefeldin A (BFA)는 락톤 항생제이자 단백질 트래피킹의 특정 억제제로, 분비 및 막 단백질이 소포체에서 골지체로 이동하는 것을 차단합니다. |
참조 |
|
적용 분야
| 방법 | 바이오마커 | 이미지 | PMID |
|---|---|---|---|
| Western blot | p53 / GRP78 |
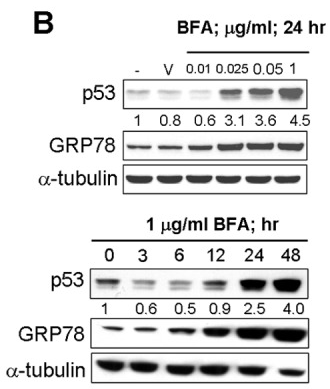
|
22859938 |
| Immunofluorescence | MTP / GBF1 ErbB3 / Calnexin FMNL1 / GM130 |
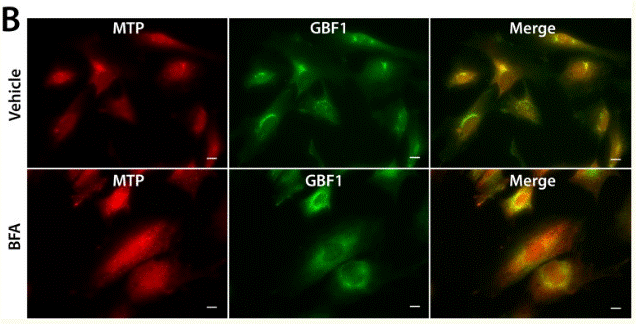
|
26267806 |
| Growth inhibition assay | Cell viability |
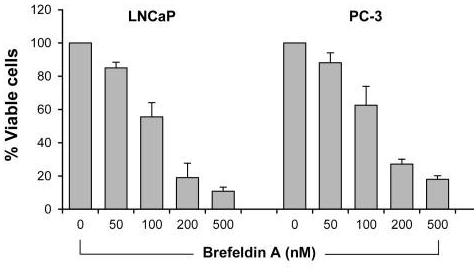
|
28462831 |
