연구용
Aspirin COX 억제제
제품 번호S3017
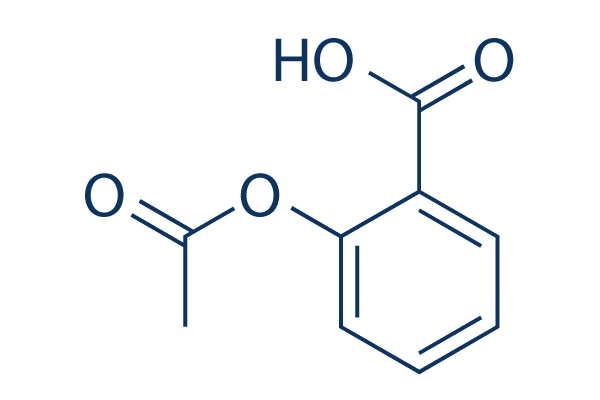
화학 구조
분자량: 180.16
품질 관리
세포 배양, 처리 및 작업 농도
| 세포주 | 분석 유형 | 농도 | 배양 시간 | 제형 | 활성 설명 | PMID |
|---|---|---|---|---|---|---|
| AGS | Function assay | 15 to 60 umol/L after | 12 hrs | Inhibition of Escherichia coli-stimulated IL-8 production in human AGS cells at 15 to 60 umol/L after 12 hrs by ELISA | 20153183 | |
| microglia cells | Antineuroinflammatory assay | 15 mins | Antineuroinflammatory activity in LPS-stimulated rat microglia cells assessed as inhibition of PMA-stimulated TXB2 release preincubated for 15 mins measured 70 mins after PMA challenge, IC50=3.12μM | 22153874 | ||
| HCT116 | Function assay | 1 mM | 6 hrs | Inhibition of TNF-alpha-induced NF-kappaB activation in human HCT116 cells at 1 mM after 6 hrs by luciferase reporter gene assay | 22154834 | |
| SKBR3 | Growth inhibition assay | 300 uM | 48 hrs | Growth inhibition of human SKBR3 cells at 300 uM after 48 hrs by alamar blue assay | 22494617 | |
| PANC1 | Growth inhibition assay | 300 uM | 48 hrs | Growth inhibition of human PANC1 cells at 300 uM after 48 hrs by alamar blue assay | 22494617 | |
| PC3 | Growth inhibition assay | 300 uM | 48 hrs | Growth inhibition of human PC3 cells at 300 uM after 48 hrs by alamar blue assay | 22494617 | |
| MDA-MB-231 | Function assay | 100 uM | 30 mins | Irreversible inhibition of COX-1 in human MDA-MB-231 cells assessed as inhibition of arachidonic acid-induced PGE2 formation at 100 uM incubated for 30 mins followed by compound washout measured 30 mins post arachidonic acid challenge by radioimmunoassay | 23651359 | |
| THP1 | Function assay | 100 uM | 30 mins | Irreversible inhibition of COX-1 in human THP1 cells assessed as inhibition of arachidonic acid-induced TXB2 formation at 100 uM incubated for 30 mins followed by compound washout measured 30 mins post arachidonic acid challenge by radioimmunoassay | 23651359 | |
| HCT116 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HCT116 cells assessed as reduction in cell viability after 48 hrs MTT assay | 26750401 | ||
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress mediated inflammation in rat H9c2 cells assessed as suppression of IL-6 in supernatant at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs by ELISA | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress mediated inflammation in rat H9c2 cells assessed as suppression of TNF-alpha in supernatant at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs in presence of leonurine by E | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress in rat H9c2 cells assessed as antioxidant activity by measuring reduction in MDA levels at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs by spectrophotometry | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress in rat H9c2 cells assessed as antioxidant activity by measuring increase in SOD activity at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs by spectrophotometry | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress in rat H9c2 cells assessed as antioxidant activity by measuring increase in catalase activity at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs by spectrophotometry | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress mediated inflammation in rat H9c2 cells assessed as suppression of TNF-alpha in supernatant at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs by ELISA | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress mediated inflammation in rat H9c2 cells assessed as suppression of IL-1beta in supernatant at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs by ELISA | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress in rat H9c2 cells assessed as reduction in MDA levels at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs in presence of leonurine by spectrophotometry | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress in rat H9c2 cells assessed as antioxidant activity by measuring increase in SOD activity at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs in presence of leonurine by spec | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress mediated inflammation in rat H9c2 cells assessed as suppression of IL-6 in supernatant at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs in presence of leonurine by ELISA | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress mediated inflammation in rat H9c2 cells assessed as suppression of IL-1beta in supernatant at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs in presence of leonurine by EL | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced cytotoxicity in rat H9c2 cells assessed as increase in cell viability at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs by MTT assay | 27575471 | |
| RAW264.7 | Antiinflammatory assay | Antiinflammatory activity in mouse RAW264.7 cells assessed as inhibition of LPS-induced nitric oxide production by measuring nitrite accumulation by Griess method, IC50=43.2μM | 28561586 | |||
| stem cells | Function assay | 100 uM | 5 days | Induction of adipogenesis in human bone marrow-derived mesenchymal stem cells assessed as increase in adiponectin production at 100 uM measured on day 5 in presence of IDX by ELISA | 29398443 | |
| DAOY | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for DAOY cells | 29435139 | |||
| Rh30 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh30 cells | 29435139 | |||
| Rh41 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh41 cells | 29435139 | |||
| peritoneal cells | Function assay | 5 hrs | Inhibition of LPS-induced PGE2 production in C57BL6 mouse peritoneal cells measured at 5 hrs time interval by ELISA, IC50=4.08μM | 30006172 | ||
| HCT8 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT8 cells after 72 hrs in presence of cinnamaldehyde by MTT assay, IC50=15.6μM | 30037494 | ||
| RPMI8226 | Cell cycle assay | 1.7 uM | 48 hrs | Cell cycle arrest in human RPMI8226 cells assessed as accumulation at G0/G1 phase at 1.7 uM in presence of bortezomib after 48 hrs by propidium iodide/RNase staining based flow cytometric method relative to bortezomib | 30590258 | |
| RPMI8226 | Cell cycle assay | 1.7 uM | 48 hrs | Cell cycle arrest in human RPMI8226 cells assessed as accumulation at S phase at 1.7 uM in presence of bortezomib after 48 hrs by propidium iodide/RNase staining based flow cytometric method relative to bortezomib | 30590258 | |
| RPMI8226 | Cell cycle assay | 1.7 uM | 48 hrs | Cell cycle arrest in human RPMI8226 cells assessed as reduction in accumulation at G2/M phase at 1.7 uM in presence of bortezomib after 48 hrs by propidium iodide/RNase staining based flow cytometric method relative to bortezomib | 30590258 | |
| HaCaT | Antipyretic assay | 100 uM | 2 hrs | Antipyretic activity in human HaCaT cells assessed as inhibition of TNFalpha-induced PGE2 production at 100 uM pre-incubated for 2 hrs before TNFalpha stimulation for 24 hrs by ELISA | 31393125 | |
| OVCAR5 | Function assay | 300 uM to 1 mM | 72 hrs | Inhibition of NAPRT in human OVCAR5 cells assessed as potentiation of NAMPT inhibitor FK866-induced cytotoxicity by measuring reduction in cell viability at 300 uM to 1 mM incubated for 72 hrs by SRB assay | ChEMBL | |
| CCRF-CEM | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human CCRF-CEM cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further | ChEMBL | |
| Jurkat | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human Jurkat cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further i | ChEMBL | |
| ML2 | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human ML2 cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further incu | ChEMBL | |
| NOMO | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human NOMO cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further inc | ChEMBL | |
| NB4 | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human NB4 cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further incu | ChEMBL | |
| NAMALWA | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human NAMALWA cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further | ChEMBL | |
| Daudi | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human Daudi cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further in | ChEMBL | |
| Raji | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human Raji cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further inc | ChEMBL | |
| ARH77 | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human ARH77 cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further in | ChEMBL | |
| RPMI8226 | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human RPMI8226 cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further | ChEMBL | |
| U266 | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human U266 cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further inc | ChEMBL | |
| NAMALWA | Function assay | 35 mg/kg | 45 days | Potentiation of NAMPT inhibitor 10 mg/kg, ip bid FK866-induced antitumor activity in human NAMALWA cells xenografted in SCID mouse assessed as increase in mouse survival at 35 mg/kg, ip bid up to 45 days | ChEMBL | |
| 클릭하여 더 많은 세포주 실험 데이터 보기 | ||||||
화학 정보, 보관 및 안정성
| 분자량 | 180.16 | 화학식 | C9H8O4 |
보관 (수령일로부터) | |
|---|---|---|---|---|---|
| CAS 번호 | 50-78-2 | SDF 다운로드 | 원액 보관 |
|
|
| 동의어 | NSC 27223, Acetylsalicylic acid, ASA | Smiles | CC(=O)OC1=CC=CC=C1C(=O)O | ||
용해도
|
In vitro |
DMSO
: 36 mg/mL
(199.82 mM)
Ethanol : 36 mg/mL Water : Insoluble |
몰농도 계산기
|
In vivo |
|||||
생체 내 제형 계산기 (투명한 용액)
1단계: 아래 정보 입력 (권장: 실험 중 손실을 고려하여 추가 동물 포함)
2단계: 생체 내 제형 입력 (이것은 계산기일 뿐 제형이 아닙니다. 용해도 섹션에 생체 내 제형이 없는 경우 먼저 당사에 문의하십시오.)
계산 결과:
작업 농도: mg/ml;
DMSO 원액 준비 방법: mg 약물 사전 용해 μL DMSO ( 원액 농도 mg/mL, 농도가 해당 약물 배치의 DMSO 용해도를 초과하는 경우 먼저 당사에 문의하십시오. )
생체 내 제형 준비 방법: 취하다 μL DMSO 원액, 다음 추가μL PEG300, 혼합하고 투명하게 한 다음 추가μL Tween 80, 혼합하고 투명하게 한 다음 추가 μL ddH2O, 혼합하고 투명하게 합니다.
생체 내 제형 준비 방법: 취하다 μL DMSO 원액, 다음 추가 μL 옥수수 기름, 혼합하고 투명하게 합니다.
참고: 1. 다음 용매를 추가하기 전에 액체가 투명한지 확인하십시오.
2. 용매를 순서대로 추가해야 합니다. 다음 용매를 추가하기 전에 이전 추가에서 얻은 용액이 투명한 용액인지 확인해야 합니다. 와동, 초음파 또는 뜨거운 물 중탕과 같은 물리적 방법을 사용하여 용해를 도울 수 있습니다.
작용 메커니즘
| Targets/IC50/Ki |
COX2
COX1
|
|---|---|
| 시험관 내(In vitro) |
Aspirin은 NF-kappa B의 활성화를 억제하여 NF-kappa B 억제제인 I kappa B의 분해를 방지하며, 따라서 NF-kappa B는 세포질에 유지됩니다. 이 화합물은 또한 형질감염된 T 세포에서 Ig kappa 증강제 및 인간 면역결핍 바이러스 (HIV) 장기말단 반복 (LTR)으로부터의 NF-kappa B 의존성 전사를 억제합니다. Aspirin과 살리실산염의 작용은 부분적으로 IKK-베타의 특이적 억제를 통해 매개되며, 이는 염증 반응의 병인에 관련된 유전자의 NF-kappaB에 의한 활성화를 방지합니다. 이 화학물질은 쥐의 일차 신경 세포 배양 및 해마 절편에서 흥분성 아미노산 글루타메이트에 의해 유발되는 신경독성에 대해 보호 효과를 가집니다. 이는 인간 제대정맥 내피세포 (HUVEC)와 호중구 [다형핵 백혈구 (PMN)]의 동시 배양 중 이전에 알려지지 않은 종류의 에이코사노이드의 경세포 생합성을 유발합니다. 이 물질은 아세틸화된 PGHS-2와 5-리포산화효소 상호작용에 의해 형성되는 독특한 종류의 에이코사노이드를 유발합니다. 이 치료는 종양괴사인자 (TNF)-알파로 처리된 3T3-L1 및 Hep G2 세포에서 Ser307의 IRS-1 인산화뿐만 아니라 JNK, c-Jun 인산화 및 IkappaBalpha 분해를 억제합니다. 이 치료는 TNF-알파에 대한 반응으로 Akt 및 라파마이신 포유류 표적 (그러나 세포외 조절 키나제 또는 PKCzeta는 아님)의 인산화를 억제합니다. 이는 TNF-알파로 전처리된 3T3-L1 지방세포에서 인슐린 유도 포도당 흡수를 회복시킵니다.
|
참조 |
|
적용 분야
| 방법 | 바이오마커 | 이미지 | PMID |
|---|---|---|---|
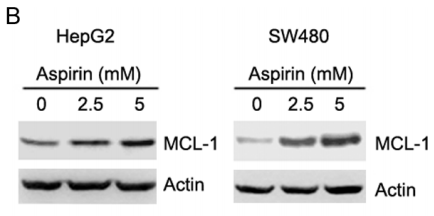
| Western blot | MCL-1 p-AKT / AKT / p-ERK / ERK p-AMPKα / AMPKα / p-ACC / ACC SHH / SMO / GLI1 / Bcl-2 / Foxm1 |
|
26918349 |
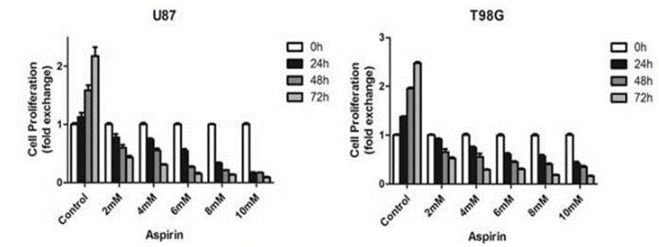
| Growth inhibition assay | Cell proliferation Cell viability |
|
28446712 |
임상시험 정보
(데이터 출처 https://clinicaltrials.gov, 업데이트 날짜 2024-05-22)
| NCT 번호 | 모집 | 조건 | 스폰서/협력자 | 시작일 | 단계 |
|---|---|---|---|---|---|
| NCT05960864 | Not yet recruiting | Ankylosing Spondylitis (AS) / Radiographic Axial SpA (r-axSpA)|Non-radiographic Axial Spondyloarthritis (Nr-axSpA)|Axial Psoriatic Arthritis (axPsA)|Acute Anterior Uveitis (AAU)|Crohn Disease (CD)|Ulcerative Colitis (UC)|Reactive Arthritis (ReA) |
Southwest Hospital China |
September 2024 | -- |
| NCT05868525 | Recruiting | Blunt Cerebrovascular Injury |
Loma Linda University |
July 2024 | Phase 4 |
| NCT06197009 | Not yet recruiting | Axial Spondyloarthritis |
Sinocelltech Ltd. |
July 1 2024 | Phase 2 |
| NCT06378398 | Not yet recruiting | Colorectal Neoplasia |
University of Michigan Rogel Cancer Center|National Cancer Institute (NCI) |
May 2024 | Early Phase 1 |
