연구용
Triapine (3-AP) DNA/RNA Synthesis 억제제
제품 번호S7470
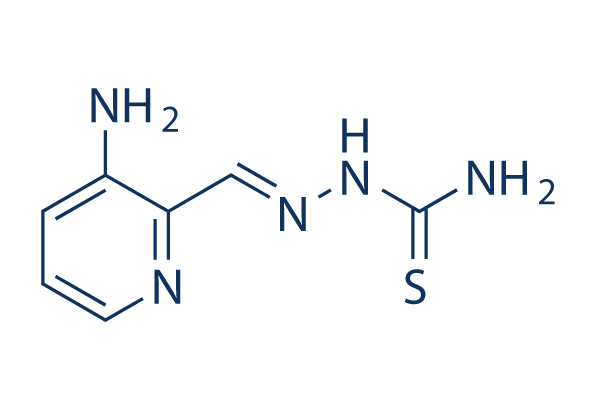
화학 구조
분자량: 195.24
품질 관리
| 관련 타겟 | HDAC PARP ATM/ATR DNA-PK WRN Topoisomerase PPAR Sirtuin Casein Kinase eIF |
|---|---|
| 기타 DNA/RNA Synthesis 억제제 | CX-5461 (Pidnarulex) B02 SCR7 Favipiravir (T-705) EED226 RK-33 BMH-21 Carmofur YK-4-279 Halofuginone |
세포 배양, 처리 및 작업 농도
| 세포주 | 분석 유형 | 농도 | 배양 시간 | 제형 | 활성 설명 | PMID |
|---|---|---|---|---|---|---|
| SK-N-MC | Antiproliferative assay | Antiproliferative activity against human SK-N-MC cells by MTT assay, IC50=0.31μM | 17142046 | |||
| SK-N-MC | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SK-N-MC cells after 72 hrs by MTT assay, IC50=0.26μM | 17602603 | ||
| SK-N-MC | Antiproliferative assay | Antiproliferative activity against human SK-N-MC cells by MTT assay, IC50=0.54μM | 17963372 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-37 cells | 18159922 | |||
| KB-3-1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human P-gp-negative KB-3-1 cells after 72 hrs by MTT assay, IC50=1.4μM | 19397322 | ||
| KB-3-1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human P-gp-negative KB-3-1 cells after 72 hrs by MTT assay, IC50=1.41254μM | 19397322 | ||
| KBV1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human P-glycoprotein-expressing KBV1 cells after 72 hrs by MTT assay, IC50=5.88844μM | 19397322 | ||
| KBV1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human P-glycoprotein-expressing KBV1 cells after 72 hrs by MTT assay, IC50=5.9μM | 19397322 | ||
| SK-N-MC | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SK-N-MC cells after 72 hrs by MTT assay, IC50=0.26μM | 19601577 | ||
| HCT116 | Dark cytotoxicity assay | 96 hrs | Dark cytotoxicity against human HCT116 cells expressing wild type p53 after 96 hrs by MTT assay, IC50=1.226μM | 24900837 | ||
| HCT116 | Photocytotoxicity assay | 24 hrs | Photocytotoxicity against human HCT116 cells expressing wild type p53 incubated for 24 hrs followed by light irradiation measured after 24 hrs by MTS assay in presence of ALA and FeCl3 | 24900837 | ||
| HCT116 | Function assay | 4 uM | 24 hrs | Induction of ROS generation in human HCT116 cells expressing wild type p53 at 4 uM after 24 hrs by spectrophotometry | 24900837 | |
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 27336684 | |||
| OHS-50 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for OHS-50 cells | 27336684 | |||
| KB-3-1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human KB-3-1 cells incubated for 72 hrs by MTT assay, IC50=3.1μM | 27336684 | ||
| KBC1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human KBC1 cells incubated for 72 hrs by MTT assay, IC50=8.9μM | 27336684 | ||
| SW480 | Function assay | 2.5 uM | 48 hrs | Induction of morphological changes in human SW480 cells assessed as induction of massive cell flattening at 2.5 uM incubated for 48 hrs by phase contrast microscopy | 27336684 | |
| HL60 | Function assay | 1 and 5 uM | 30 mins | Induction of intracellular ROS generation in human HL60 cells at 1 and 5 uM in presence of CuCl2 incubated for 30 mins by DCF-DA staining based fluorescence assay | 27336684 | |
| L1210 | Growth inhibition assay | Growth inhibition of mouse L1210 cells by MTS assay, IC50=1.3μM | 30904782 | |||
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SJ-GBM2 cells | 31614257 | |||
| WPMY-1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human WPMY-1 cells assessed as reduction in cell proliferation incubated for 72 hrs by MTT assay, IC50=4.96μM | 31614257 | ||
| GES-1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human GES-1 cells assessed as reduction in cell proliferation incubated for 72 hrs by MTT assay, IC50=5.6μM | 31614257 | ||
| HEK293 | Cytotoxicity assay | Cytotoxicity against HEK293 cells (CO-ADD:MA_007); CC50 by cell viability assay in DMEM (10% FBS) media using TC plates, by Resazurin F(560/590), CC50=2.827μM | ChEMBL | |||
| GES-1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human GES-1 cells after 72 hrs by MTT assay, IC50=5.4μM | ChEMBL | ||
| MGC803 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MGC803 cells after 72 hrs by MTT assay, IC50=9.68μM | ChEMBL | ||
| MCF7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MCF7 cells after 72 hrs by MTT assay, IC50=18.85μM | ChEMBL | ||
| SMMC7721 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SMMC7721 cells after 72 hrs by MTT assay, IC50=42.81μM | ChEMBL | ||
| 클릭하여 더 많은 세포주 실험 데이터 보기 | ||||||
화학 정보, 보관 및 안정성
| 분자량 | 195.24 | 화학식 | C7H9N5S |
보관 (수령일로부터) | |
|---|---|---|---|---|---|
| CAS 번호 | 200933-27-3 | SDF 다운로드 | 원액 보관 |
|
|
용해도
|
In vitro |
DMSO
: 39 mg/mL
(199.75 mM)
Water : Insoluble Ethanol : Insoluble |
몰농도 계산기
|
In vivo |
|||||
생체 내 제형 계산기 (투명한 용액)
1단계: 아래 정보 입력 (권장: 실험 중 손실을 고려하여 추가 동물 포함)
2단계: 생체 내 제형 입력 (이것은 계산기일 뿐 제형이 아닙니다. 용해도 섹션에 생체 내 제형이 없는 경우 먼저 당사에 문의하십시오.)
계산 결과:
작업 농도: mg/ml;
DMSO 원액 준비 방법: mg 약물 사전 용해 μL DMSO ( 원액 농도 mg/mL, 농도가 해당 약물 배치의 DMSO 용해도를 초과하는 경우 먼저 당사에 문의하십시오. )
생체 내 제형 준비 방법: 취하다 μL DMSO 원액, 다음 추가μL PEG300, 혼합하고 투명하게 한 다음 추가μL Tween 80, 혼합하고 투명하게 한 다음 추가 μL ddH2O, 혼합하고 투명하게 합니다.
생체 내 제형 준비 방법: 취하다 μL DMSO 원액, 다음 추가 μL 옥수수 기름, 혼합하고 투명하게 합니다.
참고: 1. 다음 용매를 추가하기 전에 액체가 투명한지 확인하십시오.
2. 용매를 순서대로 추가해야 합니다. 다음 용매를 추가하기 전에 이전 추가에서 얻은 용액이 투명한 용액인지 확인해야 합니다. 와동, 초음파 또는 뜨거운 물 중탕과 같은 물리적 방법을 사용하여 용해를 도울 수 있습니다.
작용 메커니즘
| Targets/IC50/Ki |
Ribonucleotide reductase
|
|---|---|
| 시험관 내(In vitro) |
Triapine (3-AP)은 야생형 KB 및 HU 저항성 KB 비인두암 세포 모두에서 리보뉴클레오티드 환원효소의 활성을 강력하게 억제하며, 일련의 암세포주에서 DNA 합성을 억제함으로써 광범위한 항종양 활성을 나타냅니다. 시험관 내에서 허혈성 신경독성과 저산소성 독성을 각각 0.35 μM 및 0.75 μM의 EC50으로 차단합니다. 이 화합물은 또한 스타우로스포린, 베라트리딘 및 글루타메이트를 포함한 신경독성 물질에 의해 유도된 세포 사멸을 억제함으로써 신경 보호 활성을 보입니다.
|
| 키나아제 분석 |
리보뉴클레오티드 환원효소 분석
|
|
CDP 환원효소는 Dowex 1-붕산 이온 교환 크로마토그래피를 사용하여 분석됩니다. 분석 혼합물은 0.02 μCi의 [14C]CDP (52.9 mCi/mmol), 3 mM 디티오트레이톨, 6 mM MgCl2, 30 mM HEPES, 5 mM ATP, 0.15 mM 비표지 CDP, 그리고 10 μL의 세포 추출물을 최종 부피 0.02 mL로 포함합니다. 반응의 배양 시간은 60분이며, 이 시간 동안 반응은 선형입니다.
|
|
| 생체 내(In vivo) |
L1210 백혈병을 앓는 쥐에서 Triapine (3-AP) (1.25 ~ 20 mg/kg)은 치명적인 독성 없이 일부 쥐에게 치료 효과가 있습니다. 이 화합물은 또한 쥐 M109 폐암 및 인간 A2780 난소암 이종이식에서 고형 종양의 성장을 억제합니다. 또한, DNA를 손상시키는 다양한 종류의 약물과의 조합은 L1210 백혈병의 시너지 억제를 유발합니다. 일시적 허혈 쥐 모델에서, i.c.v. (쥐당 50 μ) 투여 시 경색 부피를 59% 감소시키고, i.v. (1 mg/kg) 투여 시 35% 감소시킵니다.
|
참조 |
|
적용 분야
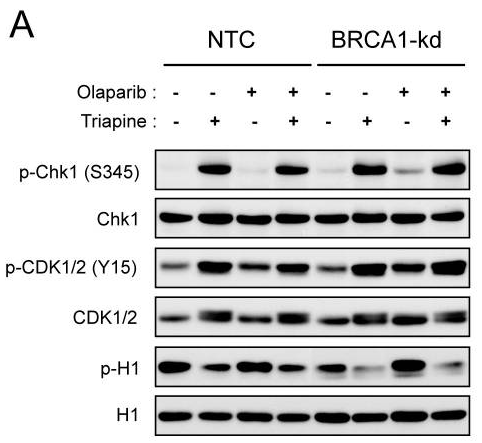
| 방법 | 바이오마커 | 이미지 | PMID |
|---|---|---|---|
| Western blot | p-Chk1 / Chk1 / p-CDK1/2 / CDK1/2 / p-H1 |
|
24413181 |
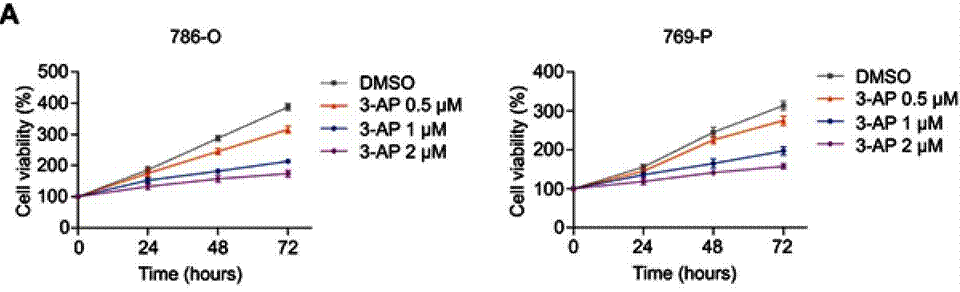
| Growth inhibition assay | Cell viability |
|
31118677 |
임상시험 정보
(데이터 출처 https://clinicaltrials.gov, 업데이트 날짜 2024-05-22)
| NCT 번호 | 모집 | 조건 | 스폰서/협력자 | 시작일 | 단계 |
|---|---|---|---|---|---|
| NCT00293345 | Completed | Anaplastic Large Cell Lymphoma|Angioimmunoblastic T-cell Lymphoma|Extranodal Marginal Zone B-cell Lymphoma of Mucosa-associated Lymphoid Tissue|Intraocular Lymphoma|Nodal Marginal Zone B-cell Lymphoma|Primary Central Nervous System Hodgkin Lymphoma|Primary Central Nervous System Non-Hodgkin Lymphoma|Recurrent Adult Burkitt Lymphoma|Recurrent Adult Diffuse Large Cell Lymphoma|Recurrent Adult Diffuse Mixed Cell Lymphoma|Recurrent Adult Diffuse Small Cleaved Cell Lymphoma|Recurrent Adult Hodgkin Lymphoma|Recurrent Adult Immunoblastic Large Cell Lymphoma|Recurrent Adult Lymphoblastic Lymphoma|Recurrent Adult T-cell Leukemia/Lymphoma|Recurrent Cutaneous T-cell Non-Hodgkin Lymphoma|Recurrent Grade 1 Follicular Lymphoma|Recurrent Grade 2 Follicular Lymphoma|Recurrent Grade 3 Follicular Lymphoma|Recurrent Mantle Cell Lymphoma|Recurrent Marginal Zone Lymphoma|Recurrent Mycosis Fungoides/Sezary Syndrome|Recurrent Small Lymphocytic Lymphoma|Small Intestine Lymphoma|Splenic Marginal Zone Lymphoma|Stage III Adult Burkitt Lymphoma|Stage III Adult Diffuse Large Cell Lymphoma|Stage III Adult Diffuse Mixed Cell Lymphoma|Stage III Adult Diffuse Small Cleaved Cell Lymphoma|Stage III Adult Hodgkin Lymphoma|Stage III Adult Immunoblastic Large Cell Lymphoma|Stage III Adult Lymphoblastic Lymphoma|Stage III Adult T-cell Leukemia/Lymphoma|Stage III Cutaneous T-cell Non-Hodgkin Lymphoma|Stage III Grade 1 Follicular Lymphoma|Stage III Grade 2 Follicular Lymphoma|Stage III Grade 3 Follicular Lymphoma|Stage III Mantle Cell Lymphoma|Stage III Marginal Zone Lymphoma|Stage III Mycosis Fungoides/Sezary Syndrome|Stage III Small Lymphocytic Lymphoma|Stage IV Adult Burkitt Lymphoma|Stage IV Adult Diffuse Large Cell Lymphoma|Stage IV Adult Diffuse Mixed Cell Lymphoma|Stage IV Adult Diffuse Small Cleaved Cell Lymphoma|Stage IV Adult Hodgkin Lymphoma|Stage IV Adult Immunoblastic Large Cell Lymphoma|Stage IV Adult Lymphoblastic Lymphoma|Stage IV Adult T-cell Leukemia/Lymphoma|Stage IV Cutaneous T-cell Non-Hodgkin Lymphoma|Stage IV Grade 1 Follicular Lymphoma|Stage IV Grade 2 Follicular Lymphoma|Stage IV Grade 3 Follicular Lymphoma|Stage IV Mantle Cell Lymphoma|Stage IV Marginal Zone Lymphoma|Stage IV Mycosis Fungoides/Sezary Syndrome|Stage IV Small Lymphocytic Lymphoma|Unspecified Adult Solid Tumor Protocol Specific|Waldenström Macroglobulinemia |
National Cancer Institute (NCI) |
June 2006 | Phase 1 |
