EGFR is a trans-membrane receptor belonging to the erbB/HER-family of RTK. EGFR exists on the cell surface and can be activated by EGF and TGF-alpha. Many important signaling cascades, like MAPK, Akt, and JNK pathways, could be the downstream of EGFR. [show the full text]
EGFR 억제제 (EGFR Inhibitors)
아이소폼 선택적 제품
| Cat.No. | 제품명 | 정보 | 제품 사용 인용 | 제품 검증 |
|---|---|---|---|---|
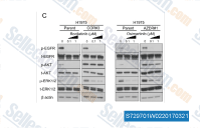
| S7297 | AZD9291 (Osimertinib) | Osimertinib (AZD9291)은 LoVo 세포에서 Exon 19 결실 EGFR, L858R/T790M EGFR 및 WT EGFR에 대해 각각 12.92, 11.44 및 493.8 nM의 IC50을 갖는 경구용, 비가역적, 돌연변이 선택적 EGFR 억제제입니다. 3상. |
|
|
| S1011 | Afatinib (BIBW2992) | Afatinib은 EGFRwt, EGFR L858R , EGFR L858R/T790M ErbB2 (HER2) 및 ErbB4 (HER4)에 대해 각각 0.5, 0.4, 10, 14, 1 nM의 IC50으로 시험관 내에서 EGFR/ErbB를 비가역적으로 억제합니다. 이 화합물은 자가포식을 유도합니다. |
|
|
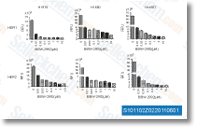
| S8724 | Lazertinib (YH25448) | Lazertinib (YH25448,GNS-1480)은 Del19/T790M, L858R/T790M, Del19, L85R 및 야생형 EGFR에 대해 각각 1.7 nM, 2 nM, 5 nM, 20.6 nM 및 76 nM의 IC50 값을 갖는 강력하고 돌연변이 선택성이 높으며 비가역적인 EGFR-TKI이며, ErbB2 및 ErbB4에 대해서는 훨씬 더 높은 IC50 값을 보입니다. |
|
|
| S1025 | Gefitinib (ZD1839) | Gefitinib은 NR6wtEGFR 및 NR6W 세포에서 Tyr1173, Tyr992, Tyr1173 및 Tyr992에 대한 EGFR 억제제로, 각각 37 nM, 37 nM, 26 nM 및 57 nM의 IC50을 가집니다. 이 화합물은 PI3K/AKT/mTOR 경로 차단을 통해 폐암 세포의 autophagy 및 apoptosis를 촉진합니다. |
|

|
| E4884 | Icotinib Hydrochloride | Icotinib Hydrochloride (BPI-2009H)는 5 nM의 IC50을 갖는 고효능 및 선택적 상피 성장 인자 수용체 티로신 키나아제 억제제(EGFR-TKI)로, 비소세포폐암(NSCLC) 치료에 사용됩니다. | ||
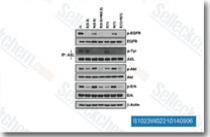
| S7786 | Erlotinib (CP-358774) | Erlotinib은 EGFR 억제제로, IC50은 2 nM이며, 인간 c-Src 또는 v-Abl보다 EGFR에 대해 >1000배 더 민감합니다. Erlotinib은 Autophagy를 유도합니다. |
|
|
| E5906New | Sunvozertinib | Sunvozertinib (DZD9008)은 ErbB (EGFR 또는 Her2), 특히 ErbB의 돌연변이 형태에 대한 경구용, 강력하고 선택적인 억제제입니다. 또한 EGFR exon 20 NPH insertion, EGFR exon 20 ASV insertion, EGFR L858R 및 T790M mutations, Her2 exon 20 YVMA 및 EGFR WT A431을 억제하며, 각각 20.4nM, 20.4nM, 1.1nM, 7.5nM, 80.4nM의 IC50 값을 가집니다. |
|
|
| S1023 | Erlotinib (CP-358774) Hydrochloride | Erlotinib HCl은 세포가 없는 분석에서 IC50가 2 nM인 EGFR 억제제로, 인간 c-Src 또는 v-Abl보다 EGFR에 대해 1000배 이상 민감합니다. |
|

|
| S2150 | Neratinib (HKI-272) | Neratinib은 무세포 분석에서 IC50가 59nM 및 92nM인 고도로 선택적인 HER2 및 EGFR 억제제입니다. KDR과 Src를 약하게 억제하며, Akt, CDK1/2/4, IKK-2, MK-2, PDK1, c-Raf 및 c-Met에 대한 유의미한 억제는 없습니다. 3상. |
|
|
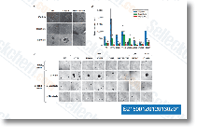
| S8362 | Tucatinib (Irbinitinib, ONT-380, Arry-380) | Tucatinib (Irbinitinib, ONT-380, ARRY-380)은 ErbB-2 (HER2라고도 함)의 경구용, 강력하고, 선택적이며, 가역적이며, ATP 경쟁적인 저분자 억제제로, 세포 기반 분석에서 ErbB-2 및 p95 HER2에 대해 각각 8 nM 및 7 nM의 IC50을 가지며, HER2에 대해 EGFR보다 약 500배의 선택성을 보입니다. 잠재적인 항종양 활성을 가지고 있습니다. |
|
|
Epidermal growth factor receptor (EGFR) was initially discovered in 1962 following the identification of the ligand EGF. Following this, the role of EGFR in protein phosphorylation and tumorigenesis has been established, and the EGF-EGFR signaling axis has consequently been the focus of research in oncology and developmental biology. Situated in the plasma membrane, EGFR has been an attractive target for anticancer therapy as it becomes activated upon ligand binding, recruiting a number of downstream molecules that leads to the activation of major pathways implicated in tumor growth, progression, and survival.[1][2]
EGFR belongs to the ErbB/HER receptor tyrosine kinase family that encompasses HER2 (ErbB2), HER3 (ErbB3), and HER4 (ErbB4).ii Each constituent of the EGFR family shares a basic domain structure including an N-terminal extracellular domain (subdivided in to domains I through IV), a transmembrane domain, an intracellular kinase domain, and a cytoplasmic C-terminal tail containing several phosphorylation sites that serve as signal transduction modules. The binding of one of several ligands to the extracellular ligand-binding domain induces receptor homo-dimerization or hetero-dimerization and results in kinase activation. Among the EGFR family, it should be noted that ErbB2 is an orphan receptor, characterized by ligand-induced hetero-dimerization with any other family member for activation. Research on ErbB3 initially did not show any intrinsic kinase activity, although recent research findings suggest otherwise.[3]
In the initial activation response of HER family constituents and similar receptor tyrosine kinases are downregulation events, involving ligand-stimulated endocytosis of occupied receptor accompanied by receptor ubiquitination and followed by lysosomal degradation of both ligands and receptors. Another possibility is that receptor tyrosine kinases are recycled from endosomes to the plasma membrane. Interestingly, HER2 and HER3 are internalized and targeted to lysosomes less efficiently than EGFR, and increased expression of HER2 or HER3 can have a dominant-negative effect on EGFR downregulation and degradation. Whether degradation and/or recycling events occur will affect the number of receptors and thus downstream signalling, profoundly changing the biological response.[2]
Ligands of EGFR include EGF, transforming growth factor-α, and heparin-binding EGF-like growth factor. Once bound to its ligand, EGFR recruits, phosphorylates and activates all of the following downstream signalling cascades: MAPK, PLC-ϒ/PKC, Ras-Raf-Mek, PI3K-Akt-mTOR/PKB and JAK2-STAT3.[1][2] EGFR can also mediate cellular processes through the physical interaction with other proteins in the absence of kinase activity or ligand activation.[1]
Several EGFR-targeting small molecule kinase inhibitors and therapeutic antibodies have been approved by the FDA to treat patients with breast cancer, colorectal cancer, non-small cell lung cancer (NSCLC), head and neck-related squamous cell carcinoma and pancreatic cancer. Despite the role that the EGFR signalling pathway plays in several downstream events that lead to tumorigenesis, currently approved EGFR-targeted therapies show only modest effect on most cancer types.[1]
Of the classes of compounds that are in clinical trials and/or are approved for clinical practice use, there are two molecular approaches to target EGFR: (1) monoclonal antibodies, directed against the external ligand-binding site of the receptor (i.e. cetuximab and panitumumab), and (2) small molecule tyrosine kinase inhibitors, directed against intracellular tyrosine kinase domain (i.e. gefitinib, erlotinib, and lapatinib). In either case, both classes of compounds target EGFR homodimers and heterodimers. Evidence of this includes the ability of erlotinib to target both EGFR and HER3. Interestingly, the impact of monoclonal antibodies targeting EGFR or HER2 is observed to impact VEGFR expression. EGFR affects VEGFR activity through the MAPK and PI3K signalling pathways and at least three different transcription factors, STAT3, Sp1 and hypoxia-inducible factors (HIFs).ii A close relative of EGFR is EGFRvIII, found to be localized on the cell-surface where it activates several signalling molecules; overexpression of EGFRvIII has been implicated in malignant gliomas. In tumorigenesis, both EGFR and EGFRvIII are observed to be involved in malignant phenotypes in human cancers.[1]
ErbB proteins are highlighted in oncology research for their ability to drive proliferation, survival, and differentiation. Their overexpression in a variety of human cancers qualifies them as an ideal target for further investigation.[3]
