PDGFR inhibitors, a class of targeted therapeutic agents designed to modulate the platelet-derived growth factor receptor (PDGFR) pathway, have emerged as a pivotal focus in cancer research and precision medicine. The PDGFR family, consisting of PDGFRα and PDGFRβ, plays a crucial role in regulating fundamental cellular processes such as proliferation, differentiation, migration, and angiogenesis. Dysregulation of PDGFR signaling, often driven by receptor overexpression, mutation, or abnormal ligand activation, is closely associated with the initiation and progression of various malignancies, making PDGFR an attractive therapeutic target. Over the past few decades, extensive scientific efforts have been devoted to understanding the molecular mechanisms underlying PDGFR-mediated oncogenesis, developing novel PDGFR inhibitors, and optimizing their clinical application.
PDGFR 억제제 (PDGFR Inhibitors)
| Cat.No. | 제품명 | 정보 | 제품 사용 인용 | 제품 검증 |
|---|---|---|---|---|
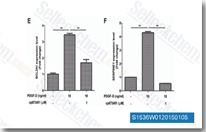
| S1536 | CP-673451 | CP-673451은 세포 유리 분석에서 10nM/1nM의 IC50을 갖는 PDGFRα/β의 선택적 억제제로, 다른 혈관신생 수용체에 대해 450배 이상의 선택성을 보이며, 혈관신생 억제 및 항종양 활성을 가집니다. |
|
|
| S8553 | Avapritinib (BLU-285) | Avapritinib (BLU-285)은 시험관 내에서 PDGFR D842V 돌연변이 활성(IC50 = 0.5 nM)과 세포 환경에서 PDGFR D842V 자가인산화를 강력하게 억제하는 소분자 키나아제 억제제이다. 또한 Kit (c-Kit)의 유사 돌연변이인 Kit (c-Kit) Exon 17의 D816V(IC50 = 0.5 nM)의 강력한 억제제이기도 하다. |
|
|
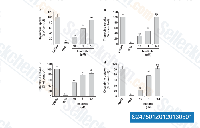
| S2475 | Imatinib (STI571) | Imatinib은 v-Abl, c-Kit 및 PDGFR을 억제하는 다중 표적 티로신 키나아제 억제제로, 세포 유리 또는 세포 기반 분석에서 IC50 값은 각각 0.6 μM, 0.1 μM 및 0.1 μM입니다. Imatinib (STI571)은 자가포식을 유도합니다. |
|
|
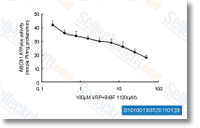
| S1010 | BIBF 1120 (Nintedanib) | Nintedanib은 VEGFR1/2/3, FGFR1/2/3 및 PDGFRα/β에 대한 강력한 삼중 혈관신생 키나제 억제제로, 세포 유리 분석에서 34 nM/13 nM/13 nM, 69 nM/37 nM/108 nM 및 59 nM/65 nM의 IC50를 가집니다. 3상. |
|
|
| S8401 | Erdafitinib (JNJ-42756493) | Erdafitinib은 잠재적인 항신생물 활성을 가진 강력하고 선택적인 경구 생체 이용 가능한 팬 fibroblast growth factor receptor (FGFR) 억제제입니다. 이 화합물은 또한 RET (c-RET), CSF-1R, PDGFR-α/PDGFR-β, FLT4, Kit (c-Kit) 및 VEGFR-2에 결합하여 세포 apoptosis를 유도합니다. |
|

|
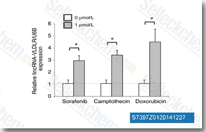
| S7397 | Sorafenib (BAY 43-9006) | Sorafenib은 세포 없는 분석에서 각각 6 nM 및 22 nM의 IC50을 갖는 Raf-1 및 B-Raf의 멀티키나아제 억제제입니다. Sorafenib은 VEGFR-2, VEGFR-3, PDGFR-β, Flt-3 및 c-KIT를 각각 90 nM, 20 nM, 57 nM, 59 nM 및 68 nM의 IC50으로 억제합니다. Sorafenib은 Autophagy 및 Apoptosis를 유도하고 항암 활성과 함께 Ferroptosis를 활성화합니다. |
|
|
| S8161 | ON123300 | ON123300은 CDK4, Ark5/NUAK1, PDGFRβ, FGFR1, RET (c-RET), Fyn에 대해 각각 3.9 nM, 5 nM, 26 nM, 26 nM, 9.2 nM, 11 nM의 IC50을 갖는 강력하고 다중 표적 키나아제 억제제입니다. |
|
|
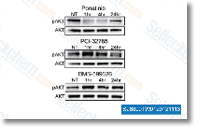
| S1490 | Ponatinib (AP24534) | Ponatinib은 Abl, PDGFRα, VEGFR2, FGFR1 및 Src의 새로운 강력한 다중 표적 억제제로, 무세포 분석에서 각각 0.37 nM, 1.1 nM, 1.5 nM, 2.2 nM 및 5.4 nM의 IC50을 가집니다. Ponatinib (AP24534)은 autophagy를 억제합니다. |
|
|
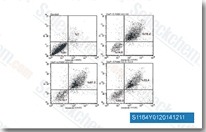
| S1164 | E7080 (Lenvatinib) | Lenvatinib은 VEGFR2(KDR)/VEGFR3(Flt-4)에 주로 작용하는 다중 표적 억제제로, IC50은 4 nM/5.2 nM이며, VEGFR1/Flt-1에 대해서는 덜 강력하고, 세포 없는 분석에서 FGFR1, PDGFRα/β에 비해 VEGFR2/3에 대해 약 10배 더 선택적입니다. Lenvatinib (E7080)은 또한 FGFR1-4, PDGFR, Kit (c-Kit), RET (c-RET)을 억제하며 강력한 항종양 활성을 보입니다. 3상. |
|
|
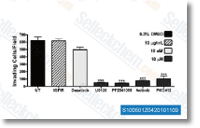
| S1005 | Axitinib (AG-013736) | Axitinib은 VEGFR1, VEGFR2, VEGFR3, PDGFRβ 및 c-Kit의 다중 표적 억제제로, 돼지 대동맥 내피 세포에서 각각 0.1 nM, 0.2 nM, 0.1-0.3 nM, 1.6 nM 및 1.7 nM의 IC50을 가집니다. |
|
|
PDGFR Receptor Biology and Signaling Pathways
The PDGFR family belongs to the receptor tyrosine kinase (RTK) superfamily, characterized by an extracellular ligand-binding domain, a single transmembrane domain, and an intracellular tyrosine kinase domain. The two main isoforms, PDGFRα and PDGFRβ, are encoded by different genes and exhibit distinct expression patterns and ligand specificities. PDGFRα binds to PDGF-A, PDGF-B, and PDGF-C, while PDGFRβ primarily interacts with PDGF-B and PDGF-D. Ligand binding induces receptor dimerization (homo- or heterodimerization between PDGFRα and PDGFRβ), which activates the intracellular tyrosine kinase domain, leading to autophosphorylation of specific tyrosine residues.
PDGFR Signaling Cascade in Normal and Pathological Conditions
The phosphorylated tyrosine residues of PDGFR serve as docking sites for various downstream signaling molecules, initiating multiple intracellular pathways that regulate cellular functions. Key signaling pathways activated by PDGFR include the Ras-Raf-MEK-ERK pathway, which promotes cell proliferation and survival; the PI3K-AKT-mTOR pathway, which regulates cell metabolism and anti-apoptotic processes; and the JAK/STAT pathway, which mediates gene transcription involved in cell growth and differentiation. In normal physiological conditions, PDGFR signaling is tightly regulated, ensuring proper tissue development, wound healing, and angiogenesis. However, in pathological states such as cancer, dysregulated PDGFR signaling disrupts this balance, leading to uncontrolled cell proliferation, enhanced cell migration and invasion, and increased angiogenesis, all of which contribute to tumor growth and metastasis.
Structural Differences Between PDGFRα and PDGFRβ
Although PDGFRα and PDGFRβ share high homology in their tyrosine kinase domains (approximately 85% amino acid sequence identity), they exhibit significant differences in their extracellular ligand-binding domains and tissue expression profiles. PDGFRα is highly expressed in embryonic tissues, and in adult tissues, it is found in fibroblasts, smooth muscle cells, and certain types of stem cells. PDGFRβ, on the other hand, is predominantly expressed in vascular smooth muscle cells, pericytes, and macrophages. These structural and expression differences contribute to the distinct roles of PDGFRα and PDGFRβ in normal physiology and disease. For example, PDGFRα mutations are frequently associated with gastrointestinal stromal tumors (GISTs), while PDGFRβ overexpression is often observed in gliomas and hematological malignancies.
PDGFR Mutation and Its Role in Cancer Oncogenesis
Mutations in the PDGFR gene are a major driver of dysregulated PDGFR signaling in cancer. These mutations can occur in the extracellular ligand-binding domain, the transmembrane domain, or the intracellular tyrosine kinase domain, leading to constitutive activation of the receptor independent of ligand binding. The identification and characterization of PDGFR mutations have provided critical insights into the molecular mechanisms of cancer development and have guided the development of targeted PDGFR inhibitors.
Common PDGFR Mutations in Human Cancers
One of the most well-characterized PDGFR mutations is the c-KIT/PDGFRα mutation in GISTs. Approximately 5-10% of GISTs harbor PDGFRα mutations, with the most common being the D842V mutation in the tyrosine kinase domain. This mutation renders PDGFRα constitutively active, promoting tumor cell proliferation and survival. Other common PDGFR mutations include the V561D mutation in the extracellular domain of PDGFRα, which enhances ligand binding affinity, and the R841W mutation in the tyrosine kinase domain of PDGFRβ, which is associated with chronic myelomonocytic leukemia (CMML). In addition, PDGFR gene amplifications and translocations have also been reported in various cancers, such as lung cancer, breast cancer, and glioblastoma, leading to receptor overexpression and hyperactivation.
Mechanisms of Mutation-Driven PDGFR Activation in Cancer
PDGFR mutations drive oncogenesis primarily by inducing constitutive receptor activation. For example, mutations in the tyrosine kinase domain, such as D842V in PDGFRα, disrupt the autoinhibitory conformation of the receptor, allowing the kinase domain to remain active even in the absence of ligand. This leads to continuous autophosphorylation of the receptor and sustained activation of downstream signaling pathways. Mutations in the extracellular domain, on the other hand, may enhance ligand binding or promote receptor dimerization, leading to increased receptor activation. In addition, PDGFR mutations can also confer resistance to certain PDGFR inhibitors, highlighting the need for personalized therapeutic strategies based on the specific mutation profile of the tumor.
Development and Evaluation of PDGFR Inhibitors for Cancer Therapy
Based on the critical role of PDGFR signaling in cancer, numerous PDGFR inhibitors have been developed and evaluated in preclinical and clinical studies. These inhibitors can be classified into two main categories: multi-targeted tyrosine kinase inhibitors (TKIs) that inhibit PDGFR along with other RTKs, and selective PDGFR inhibitors that specifically target PDGFRα or PDGFRβ. The development of PDGFR inhibitors has been guided by a deep understanding of PDGFR structure, signaling pathways, and mutation profiles, with the goal of improving efficacy and reducing off-target effects.
Preclinical Evaluation of PDGFR Inhibitors
Preclinical studies play a crucial role in the development of PDGFR inhibitors, providing insights into their mechanism of action, efficacy, and safety. In vitro studies using cancer cell lines harboring PDGFR mutations or overexpression have been used to evaluate the inhibitory activity of PDGFR inhibitors on cell proliferation, migration, and survival. In vivo studies using xenograft mouse models or genetically engineered mouse models (GEMMs) of cancer have been used to assess the antitumor efficacy of PDGFR inhibitors in vivo, as well as their pharmacokinetic and pharmacodynamic properties. For example, preclinical studies have shown that imatinib, a multi-targeted TKI that inhibits PDGFRα, PDGFRβ, and c-KIT, effectively inhibits the growth of GIST cells harboring PDGFRα mutations. Similarly, sunitinib, another multi-targeted TKI, has been shown to inhibit PDGFR signaling and suppress tumor angiogenesis in various preclinical cancer models.
Clinical Trials of PDGFR Inhibitors in Cancer Patients
Numerous clinical trials have been conducted to evaluate the safety and efficacy of PDGFR inhibitors in cancer patients. Imatinib was the first PDGFR inhibitor approved for the treatment of GISTs, and it has significantly improved the prognosis of patients with GISTs harboring c-KIT or PDGFRα mutations. However, some patients develop resistance to imatinib over time, often due to secondary mutations in PDGFRα or c-KIT. This has led to the development of second-generation PDGFR inhibitors, such as regorafenib and ripretinib, which have shown efficacy in imatinib-resistant GISTs. In addition, PDGFR inhibitors have also been evaluated in other cancers, such as lung cancer, breast cancer, glioblastoma, and hematological malignancies. For example, clinical trials have shown that sunitinib is effective in the treatment of advanced renal cell carcinoma, in part by inhibiting PDGFR-mediated angiogenesis. However, the efficacy of PDGFR inhibitors varies depending on the type of cancer and the specific PDGFR mutation profile of the tumor, emphasizing the importance of biomarker-driven patient selection.
Challenges and Future Directions in PDGFR Inhibitor Research
Despite the significant progress in PDGFR inhibitor research, several challenges remain. One of the main challenges is the development of drug resistance, which limits the long-term efficacy of PDGFR inhibitors. Secondary mutations in PDGFR, activation of alternative signaling pathways, and tumor microenvironment changes are among the main mechanisms of resistance. Another challenge is the off-target effects of multi-targeted PDGFR inhibitors, which can lead to adverse events such as hypertension, fatigue, and gastrointestinal toxicity. Future research directions include the development of more selective PDGFR inhibitors that target specific PDGFR isoforms or mutations, the identification of novel biomarkers for predicting response to PDGFR inhibitors, and the combination of PDGFR inhibitors with other therapeutic agents (such as immunotherapies) to overcome resistance and improve efficacy. In addition, further studies are needed to understand the role of PDGFR signaling in the tumor microenvironment and its interaction with immune cells, which may provide new opportunities for the development of combination therapies.
In conclusion, PDGFR inhibitors have become an important class of targeted therapeutic agents in cancer treatment, with significant progress made in understanding their mechanism of action, developing novel inhibitors, and optimizing their clinical application. The identification of PDGFR mutations and the characterization of PDGFR signaling pathways have provided critical insights into the molecular basis of cancer, guiding the development of personalized therapeutic strategies. Despite the challenges, ongoing research in PDGFR inhibitors holds great promise for improving the prognosis of cancer patients with dysregulated PDGFR signaling.
